
|

|

Frequency
The national mortality rate for Alzheimer's disease has increased
dramatically. The age-adjusted mortality rate in 1995 was 15 times the
rate in 1979 (3). Mortality data alone do not completely reflect the
number of individuals who have been affected by Alzheimer's disease.
Nonetheless, Alzheimer's disease has been added to the List of 72 Selected
Causes of Death, diseases selected by the National Center for Health
Statistics for their public health importance (3). Although Alzheimer's
disease is not usually viewed as contributing to premature mortality,
individuals with Alzheimer's or other dementias are at increased risk of
death compared to individuals without dementia who have other comparable
health conditions (4).
From study to study, estimates of the prevalence of
Alzheimer's disease vary by two- to ten-fold; when disease severity is
specified, variation is reduced (5). Using data on white individuals
pooled from 20 studies, Hy and Keller estimated that 1.7 million Americans
were affected with mild to severe Alzheimer's disease in 1996 (5). Other
researchers estimated the prevalence of Alzheimer's disease in the United
States to be between 1.1 and 4.6 million in 1997 (6). Despite this range,
public health practitioners agree that the number of individuals with
Alzheimer's disease could triple or quadruple in the next 50 years as a
result of the aging of the U.S. population (6,7). If new treatments that
would both delay onset and progression of disease are developed, the
number of affected individuals would double by 2050 rather than quadruple
(7). Dementia can also occur as a result of vascular disease, HIV
infection, Parkinson's disease, and other diseases.
Individuals with
dementia account for nearly 58,000 hospitalizations each year in New York
State. In 43% of the hospitalizations among individuals with dementia, the
individual is discharged or returned to a skilled nursing facility (P.P.L.,
unpublished data, 2003).
Severity
Dementia can result in a diminished quality of life not only for the
affected individual but also for the family. Individuals with
Alzheimer's disease experience problems with memory and may also have
other cognitive impairments such as difficulties with language, inability
to recognize and name objects or people, and loss of judgment and
problem-solving skills (8). Behavioral symptoms such as agitation,
wandering, and inappropriate behavior and psychological problems including
delusions or hallucinations may also occur (9). Individuals with dementia
are 5 times more likely to be admitted to a nursing home than others of
the same age (4). One fourth of individuals with mild Alzheimer's disease
progress to severe Alzheimer's within 5 years; another quarter die in this
time period (10).
In a recent study of hospitalizations in New York State,
the majority of hospitalizations for individuals with a diagnosis of
dementia occurred on an emergency basis (86%). Many of the
hospitalizations were due to serious illness such as pneumonia and
pneumonitis (13%), septicemia (4%), and heart failure (4%) (P.P.L.,
unpublished data, 2003).
Disparities
In the United States African American and Caribbean Hispanic
individuals have been found to be at twice the risk of Alzheimer's disease
as white individuals (11). Data from Medicare claims supports high risk of
other dementias in African American individuals as well (12).
In
hospitalizations in New York State, the number of hospitalizations among
women with Alzheimer's disease was twice the number among men. Nearly half
of New York hospitalizations that included a diagnosis of dementia among
women occurred for women over the age of 84; for men, a third of New York
hospitalizations that included a diagnosis of dementia occurred among men
over the age of 84. African American individuals accounted for 10% of all
hospitalizations among New York State's hospitalized individuals over the
age of 65, but were 14% of the New York hospitalizations that included a
diagnosis of dementia (P.P.L., unpublished data, 2003).
Costs
Estimates of the cost of Alzheimer's disease vary widely — but all are
high (13). Medicare and Medicaid expenditures are 50% to 100% higher among
recipients with Alzheimer's disease compared with other recipients (14,15).
Medicare beneficiaries with Alzheimer's disease are hospitalized more
frequently and stay in the hospital longer than other Medicare
beneficiaries but receive fewer diagnostic and therapeutic procedures
(16). Care for individuals with vascular dementia is even more costly
(17). Alzheimer's disease and other dementias are suggested as major
hidden contributors to the high costs of treating other chronic diseases
such as diabetes and cancer (17). The value of lost productivity among
caregivers substantially exceeds the direct medical costs among
community-dwelling individuals with Alzheimer's disease (18).
Preventability
Medical advances are offering the opportunity to slow the
progress of Alzheimer's disease. The U.S. Food and Drug Administration has
approved a number of pharmacological interventions that improve cognitive
function in Alzheimer's patients; more are being investigated (8).
Preventing or controlling the underlying disease can prevent dementias
such as vascular dementia or dementia resulting from HIV.
Public Interest
Dementia does not appear in the popular news media to
the degree that other health issues, such as breast cancer, do. This could
be due to misconceptions about the inevitability of cognitive decline with
aging.
Challenges in Surveillance of Dementias
Level of Usefulness
The legislative intent of the law establishing the Registry was to
create a registry as a resource for describing the magnitude of dementia
in New York State and supporting research on dementia.
The Registry has contributed to its research mission in a number of ways:
- A study of physician diagnosis of dementia established baseline data
for Healthy People 2000: National Health Promotion and Disease
Prevention Objectives and was used to target educational interventions
and teleconferences;
- Creutzfeldt-Jakob Disease (CJD) surveillance linking Registry, death
records, and hospital data is conducted as part of a national effort
to more rapidly identify potential instances of CJD and variant CJD in
New York State; and
- Registry data was used in a study of caregivers' need for health and
human services conducted by the Institute of Gerontology, University
at Albany, State University of New York (19,20).
Many of the challenges described in this paper have limited the
usefulness of the Registry in the past and are currently being addressed.
System Attributes
The CDC guidelines list 9 attributes for evaluating a surveillance
system (1). These include simplicity, flexibility, data quality,
acceptability, sensitivity, positive predictive value, representativeness,
timeliness, and stability. A brief review of these attributes as they
apply to the Registry follows. A focus on data quality will serve to
clarify the challenges faced in conducting surveillance of dementia.
Simplicity
Simplicity refers to structure and ease of operation of the surveillance
system (1). Simplicity is enhanced by use of other existing sources of
reporting data. Figure 1 illustrates the anticipated flow of information to the
Registry from nursing homes, and Figure 2 shows the flow from hospitals.
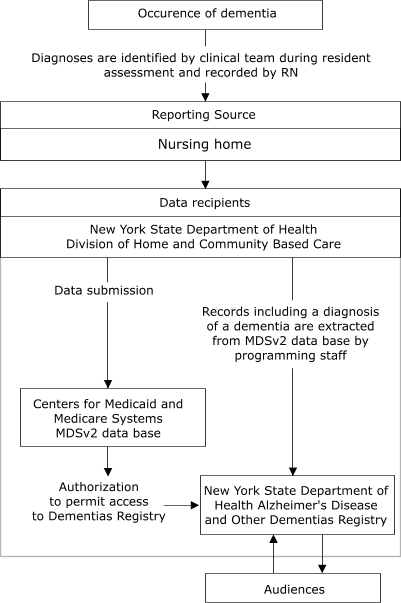
Figure 1.
Proposed flow of data from nursing homes to New
York State Department of Health Alzheimer's Disease and Other Dementias
Registry, 2003. MDSv2 indicates Minimum Data Set 2.0, Centers for Medicare
and Medicaid Services, U.S. Department of Health and Human Services.
Permission to use MDSv2 data for Dementias reporting is being sought by
the Registry.
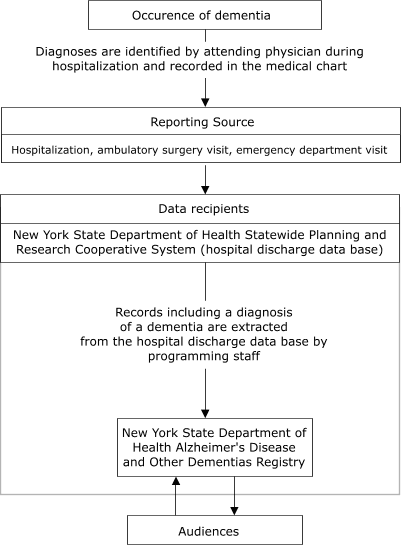
Figure 2.
Flow of data from hospitals to New York
State Department of Health Alzheimer's Disease and Other Dementias
Registry, 2003.
Flexibility
Flexibility refers to how quickly a system can adapt to changing
information needs or operating conditions (1). Because dementia is a
chronic disease, information needs and operating conditions change slowly,
and flexibility is not a critical issue. Nevertheless, the Registry seeks
to maximize flexibility by obtaining data from existing resources, rather
than collecting its own data in isolation. The process of procuring data
from other sources has not been difficult, but the process of obtaining
permissions and developing programs to extract data has been
time-consuming. Technology permits extraction of high-quality data from
other systems, facilitating operation of the Registry with little
staffing, but the Registry is only as flexible as its existing data
sources.
Data quality
Data quality reflects the completeness and validity of data recorded (1).
Data quality — specifically, completeness and coding accuracy — is the
primary challenge to the Registry. The Registry relies on 2 primary data
sources for completeness of information: hospitals and nursing homes.
Between 1986 and early 2003, hospitals reported data on paper forms sent
directly to the Registry. During this time, hospital reporting via paper
forms yielded approximately 20,000 reports each year. In comparison, New
York State's hospital discharge database received more than 50,000 medical
records that include a diagnosis of dementia each year. The hospital
discharge database collects information on up to 15 ICD-9-CM diagnoses
from over 2.4 million hospitalizations statewide each year. In spring
2003, the Registry eliminated paper reporting from hospitals, converting
to computerized extraction of hospitalizations with a dementia diagnosis
from the hospital discharge database. This eliminated the need for
hospital staff to make separate reports to the Registry, and it also
eliminated the need for the Registry to spend resources on data entry. The
hospital discharge database locates additional reports on individuals with
dementia, and it maintains quality control mechanisms and important
hospital relationships.
Use of the hospital discharge database does not eliminate all concerns
about completeness of hospital reporting. Completeness also depends on
dementia diagnoses being recorded in medical records in the first place.
Dementia diagnoses may be underreported for several reasons. The first
reason is diagnostic uncertainty. Unlike a cancer diagnosis, which is based on
laboratory pathology, Alzheimer's disease and most other dementias are
diagnosed clinically. Although it is commonly believed that Alzheimer's
disease can only be definitively diagnosed at autopsy, practice parameters
developed by the American Academy of Neurology indicate that Alzheimer's
disease can be diagnosed clinically with good reliability (21). National Center for Health
Statistics guidelines call for coding discharge diagnoses that are suspected, possible, or probable
as if the condition "existed or was established" (22). Because of
uncertainties in assigning a diagnosis, physicians may hesitate to record
a clinical diagnosis or possible diagnosis of dementia in the medical
record. Second, general practitioners have been found to delay diagnoses
of dementia because of embarrassment about communicating the diagnosis
(23). The degree to which doctors hesitate is unknown. Third, financial
disincentives for reporting dementia have also existed. Insurers have
denied payment for services such as physical, occupational, or speech
therapy to patients with Alzheimer's disease in the belief that patients
could not benefit from therapy (23,24). Automatic denial based solely on
a diagnosis of dementia has been prohibited since September 2001 (24).
Fourth, most individuals with dementia are hospitalized for illnesses
other than dementia, such as cardiovascular disease or respiratory disease
(P.P.L., unpublished data, 2003). Failure to record diagnoses may occur
because of clinical attention to the primary reason for
hospitalization. The degree to which hospitals vary in recording secondary
diagnoses is unknown.
Nursing homes provide another source of information on patients with
dementia. A diagnosis of dementia is likely to be well recorded in nursing
home data because nursing homes are mandated to assess residents'
abilities and limitations frequently to provide a responsive plan of care.
Higher reimbursement rates for residents who require more care may also
encourage complete reporting of dementia by nursing homes. Approximately
200,000 assessments are performed each year for nursing home residents
described as having dementia; the evaluations are recorded in the Centers
for Medicare and Medicaid Services' Minimum Data Set. A registered nurse
coordinates the assessment and certifies that the assessment form is
complete. Other licensed health professionals, such as the attending
physician, social workers, dietitians, and physical therapists may be
assigned to complete relevant sections of the assessment instrument.
The Registry is now pilot-testing the ability of this computerized
database to replace paper reporting from nursing homes. Preliminary data
show that only one third of nursing homes that reported using the Minimum
Data Set also completed paper Registry reporting forms. Use of the Minimum
Data Set to retrieve information on nursing home residents with dementia
would eliminate the need for nursing home staff to report data to 2
systems, and Registry staff would not need to enter data separately.
Administrators of the nursing home data system monitor quality control,
provide training on filling out forms, and maintain relationships with
nursing homes.
Accuracy in coding the dementia diagnosis is another important component
of data quality. Diagnoses in medical records are currently coded and
reported under the ICD-9-CM coding system. Conceptualization of
Alzheimer's disease and other dementias has evolved over time (25), and
coding reflects this evolution. Under the ICD-9-CM system, dementias are
grouped with psychotic mental disorders, while Alzheimer's disease is
classified as a nervous system disorder. Consequently, reporting 2 codes
is necessary for some dementias: one to document dementia symptoms and
another to document the disease responsible for the dementia, such as
Alzheimer's or Parkinson's disease. ICD-9-CM terminology further
complicates coding accuracy because it has not kept pace with terms used
by physicians and neurologists in discussing dementias. Recent practice
parameters from the American Academy of Neurology discuss criteria for
diagnosing Alzheimer's disease, vascular dementia, dementia with Lewy
Bodies, and frontotemporal dementia (21). Several ICD-9-CM codes document
Alzheimer's disease. In contrast, no ICD-9-CM codes existed prior to
October 2003 for frontotemporal dementia or dementia with Lewy Bodies
(26). As a result, the consistency of coding dementias may vary from
facility to facility, given differences between how physicians describe
dementia and how the coding system offers options. A study of
hospitalizations that included a diagnosis of dementia found various
dementia diagnoses recorded for the same patient both over time and within
the same hospital stay (P.P.L., unpublished data, 2003). The Medical
Economics and Management Subcommittee of the American Academy of Neurology
is working to develop an ICD-9-CM Dementia coding index to address some of
these issues (written communication, Gina Gjorvad, American Academy of
Neurology, 31 July 2003).
Acceptability
Acceptability indicates the willingness of persons and organizations to
participate in the surveillance system (1). Use of existing sources of
data from hospitals and nursing homes clearly enhances acceptability of
the Registry. Uncertainty about diagnosing dementia and a possible
perceived stigma or embarrassment among physicians may pose a significant
obstacle to acceptability, but the extent of significance is unknown. A
recent review of hospitalizations found one third of men and nearly half
of women hospitalized with a recorded dementia diagnosis were over age 85,
suggesting that physicians may be more willing to record a dementia
diagnosis for older patients (P.P.L, unpublished data, 2003).
Sensitivity
Sensitivity refers to the proportion of disease cases detected by the
surveillance system as well as the ability to detect outbreaks, including
the ability to monitor changes in the number of cases over time (1).
Historically, the majority of Registry reports have come from hospitals
and nursing homes. The mandate to report Alzheimer's disease and other
dementias is not contained in the same section of New York State public
health law as communicable diseases. Direct reporting of communicable
diseases is a traditional public health activity that is familiar to
physicians and county health departments. Reporting of dementia is less
familiar to physicians than reporting communicable diseases, particularly
since county health departments are not used as a conduit of dementia data
to the state. Resources have not permitted the degree of outreach and
education necessary to inform physicians of their duty to report or to
monitor completeness and accuracy of their reporting.
As a result, the surveillance system is sensitive enough to monitor
changes over time, but is most sensitive to individuals with dementia who
are frail or have comorbid conditions and least sensitive to individuals
in relatively good health or those diagnosed in the early stages of
dementia. The idea of representativeness is discussed further below.
Predictive Value Positive
Predictive value positive is the proportion of individuals reported with
diesease that actually have disease (1). Factors that limit the recording of
dementia diagnoses in medical charts also make false-positive reports of
dementia unlikely.
Representativeness
Representativeness is a measure of how accurately the surveillance system
describes the occurrence and distribution of a health-related event over
time (1). The Registry represents individuals with dementia who are frail
or who have comorbid conditions better than it represents individuals in relatively good
health or individuals diagnosed at early stages of disease, mainly
because reports are made primarily from hospitals and nursing homes. Medical
advances are making early diagnoses more possible. As more individuals
receive early diagnoses outside the hospital or nursing home setting, the
current system of surveillance will not be able to capture these
diagnoses, and the system will become less representative of the
population with dementia.
Timeliness
Timeliness reflects the speed between surveillance system steps (1).
Timeliness is less important to surveillance of chronic diseases such as
dementia compared to surveillance of communicable diseases, which demands
a rapid public health response (1). Long-range planning of support
services is enhanced by information on statistical trends in Alzheimer's
disease and other dementias. The NYSDOH
provides small grants to Alzheimer's Disease Assistance Centers and
Alzheimer's Disease Community Service Programs in New York State.
Assistance Centers provide diagnosis, assessment and treatment for
patients, educational/training services for health care professionals,
care planning for the patient and family and serve as a clearinghouse for
information on dementia. Community Service Programs provide respite care,
caregiver training, support group services, and information and referral
to patients and families. Information on trends in Alzheimer's disease and
other dementias will enhance long-range planning of services by these and
other groups. Staff members from the Registry have participated in annual
meetings of these organizations.
Stability
Stability refers to the reliability (i.e., the ability to collect, manage,
and provide data without failure) and availability (i.e., the ability to
be operational when needed) of a surveillance system (1). Because
timeliness is not crucial to dementia surveillance, the Registry is
considered to be a stable system — that is, reliable and available with
given resources.
Back to top
Conclusions and Recommendations
This article has highlighted data quality as the main challenge to
surveillance of dementias in New York State. Data quality is likely to be
the primary challenge to other surveillance systems focusing on dementias.
A system that obtains reports from existing hospital and nursing home
databases can improve completeness of reporting, but such a system may be
biased toward documenting illness in frail individuals with other health
conditions as well as those living in nursing homes. Terminology reflected
in ICD-9-CM coding has not kept pace with terms used by physicians and
neurologists in discussing dementias. The accuracy of coding dementias in
medical records from facility to facility is unknown; differences exist
between how dementias are described in medical charts and how they are
translated into coding choices.
A number of additional activities may improve completeness and accuracy of
dementia reporting from nursing homes and hospitals. These recommended
activities include:
- Educating physicians on how to diagnose and code for dementia
accurately once American Academy of Neurology guidelines are finalized.
- Examining patterns of dementia diagnoses reported longitudinally for a
sample of patients from different sources to assess the consistency of
dementia diagnoses.
- Enlisting the state's professional society for medical information
management as a partner to highlight the importance of completely
recording diagnoses of dementia and to help make informed recommendations
about best practices in coding.
As medical advances lead to earlier diagnoses of dementia in individuals
without other health conditions, we will need to develop better mechanisms
for capturing data from organizations other than hospitals and nursing
homes. A number of approaches could be tested:
- Conduct a capture-recapture study using existing data sources to
estimate the proportion of individuals with dementias missed in current
surveillance. Such a study was recently undertaken by the South Carolina
Alzheimer's Disease Registry; use of capture-recapture methodology was
shown to identify 25% more individuals with dementia in South Carolina
for an overall prevalence of 14% among individuals 65 years of age and
older (27).
- Partner with neurology practices and community-based organizations
providing support services to individuals with Alzheimer's disease to
study the proportion of individuals with dementia who go unreported.
- Explore the utility of other databases, such as Medicare encounter
data.
Despite the limitations of dementia surveillance, surveillance data are
useful to individuals and agencies involved in monitoring trends,
conducting research, or planning future services for an aging population.
To that end, the Registry will publish its first report on
hospitalizations that include a diagnosis of dementia in New York State.
The report is scheduled
for release at the end of 2003. Its intended audience includes public
health practitioners and planners, clinicians, Alzheimer's Disease
Assistance Centers, Alzheimer's Disease Community Service Programs, and
reporting facilities. By studying hospitalizations, the Registry can begin
to characterize the experience of New York's frailest residents coping
with dementias and other illnesses.
Back to top
Author Information
Corresponding Author: Patricia P. Lillquist, MSW, Alzheimer's Disease and
Other Dementias Registry, Bureau of Chronic Disease Epidemiology and
Surveillance, New York State Department of Health, Room 565, Corning
Tower, Empire State Plaza, Albany, New York 12237. Phone: 518-473-7817. E-mail: PPL02@health.state.ny.us
Back to top
References
- Centers for Disease Control. Updated guidelines for evaluating public
health surveillance systems: recommendations from the Guidelines Working
Group. MMWR Morb Mortal Wkly Rep 2001;50 (No. RR-13):1-35.
- NY Pub Health, Article 20, §2000-2004 (McKinney 2003).
- Hoyert DL, Rosenberg HM.
Alzheimer's disease as a cause of death in the
United States. Public Health Rep 1997;112:497-505.
- Eaker ED, Vierkant RA, Mickel SF.
Predictors of nursing home admission
and/or death in incident Alzheimer's disease and other dementia cases
compared to controls: a population-based study. J Clin Epidemiol 2002;55:462-8.
- Hy LX, Keller DM.
Prevalence of AD among whites. Neurology 2000;55:198-204.
- Brookmeyer R, Gray S, Kawas C.
Projections of Alzheimer's disease in
the United States and the public health impact of delaying disease onset.
Am J Public Health 1998;88:1337-42.
- Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, et
al.
The public health impact of Alzheimer's disease, 2000-2050: potential
implication of treatment advances. Annual Rev Public Health 2002;23:213-31.
- Dugué M, Neugroschl J, Sewell M, Marin D.
Review of dementia. Mt Sinai
J Med 2003;70 (1):45-53.
- Parnetti L, Amici S, Lanari A, Gallai V.
Pharmacological treatment of
non-cognitive disturbances in dementia disorders. Mech Ageing Dev 2001;122:2063-9.
- Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KS,
et al.
Measuring Alzheimer's disease progression with
transition probabilities: estimates from CERAD. Neurology 2001;57:957-64.
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al.
Incidence of AD in
African-Americans, Caribbean Hispanics, and Caucasians in northern
Manhattan. Neurology 2001 Jan 9;56 (1):49-56.
- Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA.
Racial
differences in the diagnosis of dementia and in its effects on the use and
costs of health care services. Psychiatr Serv 2003 Jan;54 (1):92-6.
- Bloom BS, de Pouvourville N, Straus WL.
Cost of illness of Alzheimer's
disease: how useful are current estimates? Gerontologist 2003
Apr;43 (2):158-64.
- Sloan FA, Taylor DH Jr.
Effect of Alzheimer disease on the cost of
treating other diseases. Alzheimer Dis Assoc Disord 2002
Jul-Sep;16 (3):137-43.
- Martin BC, Ricci JF, Kotzan JA, Lang K, Menzin J.
The net cost of
Alzheimer disease and related dementia: a population-based study of
Georgia Medicaid recipients. Alzheimer Dis Assoc Disord 2000
Jul-Sep;14 (3):151-9.
- Fillenbaum G, Heyman A, Peterson B, Pieper C, Weiman AL.
Frequency and
duration of hospitalization of patients with AD based on Medicare data: CERAD XX. Neurology 2000;54:740-3.
- Fillit H, Hill J.
The costs of vascular dementia: a comparison with
Alzheimer's disease. J Neurol Sc 2002;203-204:35-9.
- Small GW, McDonnell DD, Brooks RL, Papadopoulos G.
The impact of
symptom severity on the cost of Alzheimer's disease. J Am Geriatr Soc
2002;50:321-7.
- Toseland RW, McCallion P, Gerber T, Dawson C, Cieryic S, Guilamo-Ramos
V. Use of health and human services by community-residing people with
dementia. Soc Work 1999 Nov;44 (6):535-48.
- Toseland RW, McCallion P, Gerber T and Banks S.
Predictors of health
and human services use by persons with dementia and their family
caregivers. Soc Sci Med 2002 Oct;55 (7):1255-66.
- Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin
N, et al.
Practice parameter: diagnosis of dementia
(an evidence-based review): Report of the Quality Standards Subcommittee
of the American Academy of Neurology. Neurology 2001;56:1143-53.
- National Center for Health Statistics. ICD-9-CM Official Guidelines
for Coding and Reporting, October 2002. Available from: URL: http://www.eicd.com/Guidelines/Default.htm*
- van Hout H, Vernooij-Dassen M, Bakker K, Blom M, Grol R.
General practitioners on dementia: tasks, practices and obstacles. Patient Educ Couns
2000;39 (2-3):219-25.
- Centers for Medicare & Medicaid Services.
Program Memorandum
Intermediaries/Carriers Transmittal AB-01-135, CMS-Pub 60AB, September 25,
2001.
- White L.
Alzheimer's Disease: The evolution of a diagnosis. Public
Health Rep 1997;112 (6):495-6.
-
ICD-9-CM Coordination and Maintenance Committee Meeting, Volumes 1 and
2, Diagnostic Presentations, December 6, 2002.
- Sanderson M, Benjamin JT, Lane MJ, Cornman CB, Davis DR.
Application of capture-recapture methodology to estimate the prevalence of
dementia in South Carolina. Ann Epidemiol 2003 Aug;13 (7):518-24.
*URLs for nonfederal organizations are provided solely as a
service to our users. URLs do not constitute an endorsement of any organization
by CDC or the federal government, and none should be inferred. CDC is
not responsible for the content of Web pages found at these URLs.
Back to top
|
|
