Resources for Health Professionals
Diagnosis
Definitive diagnosis rests on the observation of trypanosomes by microscopy.
In T. b. rhodesiense infection, the identification of suspected cases relies on the clinical presentation and a history of exposure. The level of parasitemia is relatively high, particularly in the first stage of disease, and trypanosomes can be found in blood. In centrifuged blood, the parasite sediments just above the white cells, and examination of buffy coat will increase sensitivity. Slides stained with Giemsa can be used, but it is easiest to find the parasite by microscopic examination of fresh wet preparations, because the trypanosomes are motile and attract the eye. Delay between sampling and microscopy should be minimized, because trypanosomes will lose motility within a few hours. Parasites can also be found in fluid expressed in trypanosomal chancres and in lymph node aspirates. Serologic testing is not used for the diagnosis of T. b. rhodesiense infection.
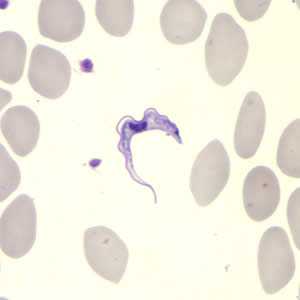
Trypanosoma brucei ssp. in a thin blood smear stained with Giemsa. Credit: DPDx
Detecting trypanosomes in T. b. gambiense infection is more difficult. The card agglutination test for trypanosomiasis/T. b. gambiense is a serologic screening test used for mass population screening in endemic areas of Africa. It is not available in the U.S., however, CDC can provide information for testing in Europe. The test is not specific enough for confirmation of infection, but it is helpful in identifying suspect cases. For parasitologic confirmation, a posterior cervical lymph node (if present) is punctured and the fluid examined. The yield in lymph node examination varies from about 40% to 80%. Trypanosomes can also be found in blood, however, the yield is low, and concentration techniques (e.g. buffy coat examination, miniature anion-exchange centrifugation technique) are helpful. Serial examinations on consecutive days may be needed.
Treatment decisions are based on the stage of the disease. Every patient diagnosed with African trypanosomiasis must undergo a lumbar puncture for the examination of CSF. The most widely used criteria for defining second stage disease are the observation of trypanosomes in CSF or a white cell count of 6 or higher. Other indications of second stage disease include elevated protein and an increase in nonspecific IgM in CSF.
Diagnostic assistance for African trypanosomiasis is available through DPDx .
Treatment
Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis. Choice of therapy depends on the infecting subspecies of the parasite and on the disease stage. The first line drugs for both first and second stage disease are highly effective. Pentamidine, given by intravenous infusion over 2 hours or by intramuscular injection, is used to treat first stage T. b. gambiense infection. It is generally well tolerated, but adverse reactions of hypoglycemia, injection site pain, diarrhea, nausea and vomiting occur. Suramin is used to treat first stage T. b. rhodesiense. Suramin is also effective against T. b. gambiense, but it is not often used because severe reactions occur in persons who are co-infected with Onchocerca volvulus. Adverse reactions to suramin are frequent, but usually mild and reversible. These include drug rash, nephrotoxicity, and peripheral neuropathy. In rare instances, suramin administration results in a hypersensitivity reaction, and, for this reason, a small test dose is usually given prior to the full first dose.
Second stage T. b. gambiense is treated with eflornithine, which is given in 4 intravenous infusions daily for 14 days. Adverse effects of eflornithine include bone marrow suppression, gastrointestinal symptoms, and seizures. Eflornithine is highly effective, but the difficulty in administering 4 infusions daily in rural African facilities has led to the use of eflornithine (dosed less frequently) in combination with nifurtimox. The efficacy of the combination regimen appears to be at least as high as eflornithine monotherapy. Eflornithine is not effective against T. b. rhodesiense and it is not recommended for treating the East African form of the disease. Melarsoprol, an organoarsenic compound, is the only drug available for treating second stage T. b. rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. An encephalopathic reaction occurs in 5-10% of patients with a case-fatality rate of approximately 50% when it occurs. Prednisolone is often given to patients who are being treated with melarsoprol to reduce the risk of encephalopathy. Other adverse reactions observed with melarsoprol include skin reactions, gastrointestinal upset, and peripheral neuropathy. Intravenous injections of melarsoprol are painful and can cause phlebitis. The drug is administered by use of lengthy and complicated dosing schedules, however, an abbreviated 10-day regimen appears promising.
| Species | Drug of choice | Adult Dosage | Pediatric Dosage |
|---|---|---|---|
| T. b. rhodesiense, hemolymphatic stage | Suramin1 | 1 gm IV on days 1, 3, 7 ,14, and 212 | 20 mg/kg IV on days 1, 3, 7, 14, and 213 |
| T. b. rhodesiense, CNS involvement | Melarsoprol4 | 2-3.6 mg/kg/day IV x 3 days.5 After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. | 2-3.6 mg/kg/day IV x 3 days.5 After 7 days, 3.6 mg/kg/day x 3 days. Give a 3rd series of 3.6 mg/kg/d after 7 days. |
| T. b. gambiense, Hemolymphatic stage | Pentamidine6 | 4 mg/kg/day IM or IV x 7-10 days | 4 mg/kg/day IM or IV x 7-10 days |
| T. b. gambiense, CNS involvement | Eflornithine7 | 400 mg/kg/day in 4 doses x 14 days | 400 mg/kg/day in 4 doses x 14 days |
Pentamidine is available for human use in the United States.
Eflornithine is available for human use in the United States through the Centers for Disease Control and Prevention (CDC).
There is no test of cure for African trypanosomiasis. Patients should be followed with a lumbar puncture every 6 months (or sooner, if symptoms return) for 2 years after treatment to detect a relapse should it occur.
For treatment advice and to obtain suramin, melarsoprol, or eflornithine, physicians should contact the Division of Parasitic Diseases and Malaria (telephone, 404-718-4745; email, parasites@cdc.gov).
More on: Human African trypanosomiasis, WHO
- Pentamidine is also effective against T. b. rhodesiense in the hemolymphatic stage, but suramin may have somewhat higher efficacy.
- A test dose of 100 mg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- A test dose of 2 mg/kg should be given prior to the first dose and the patient should be monitored for hemodynamic stability.
- Corticosteroids have been used to prevent melarsoprol encephalopathy.
- The dose of melarsoprol is progressively increased during the first series.
- Suramin is also effective against T. b. gambiense in the hemolymphatic stage.
- Eflornithine (400 mg/kg/d IV in 2 doses x 7 days) given in combination with oral nifurtimox (15 mg/kg/d x 10 days) is also highly effective against T. b. gambiense with CNS involvement (Priotto G et al. Lancet 2009:374; 56-64). Nifurtimox is not FDA-approved for this indication.
This information is provided as an informational resource for licensed health care providers as guidance only. It is not intended as a substitute for professional judgment.
Eflornithine
Note on Treatment in Pregnancy
Data on the use of eflornithine in pregnant women are limited, and risk to the embryo-fetus is unknown. Eflornithine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Note on Treatment During Lactation
It is not known whether eflornithine is excreted in breast milk. Eflornithine should be used with caution in breast-feeding women.
Note on Treatment in Pediatric Patients
The safety of eflornithine in children has not been established. Eflornithine is not approved by the Food and Drug Administration (FDA) for use in pediatric patients. Eflornithine is listed for the treatment of 1st stage African trypanosomiasis in Trypanosoma brucei gambiense infection on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
Pentamidine
Note on Treatment in Pregnancy
Pentamidine is in pregnancy category C. Data on the use of pentamidine in pregnant women are limited, and risk to the embryo-fetus is unknown. Pentamidine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Note on Treatment During Lactation
It is not known whether pentamidine is excreted in breast milk. The World Health Organization (WHO) classifies pentamidine as compatible with breast-feeding, although data on the use of pentamidine during lactation are limited. Pentamidine should be used during lactation only if the potential benefit of therapy to the mother justifies the potential risk to the infant.
Note on Treatment in Pediatric Patients
Intravenous and intramuscular pentamidine have a similar safety profile in children age 4 months and older as in adults. Pentamidine is listed as a medicine for the treatment of 1st stage African trypanosomiasis infection (Trypanosoma brucei gambiense) on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
- Page last reviewed: July 15, 2016
- Page last updated: August 10, 2016
- Content source:


 ShareCompartir
ShareCompartir