Resources for Health Professionals
Diagnosis
The gold standard test for the diagnosis of onchocerciasis remains the skin snip biopsy. The biopsy is performed using a sclerocorneal biopsy punch or by elevating a small cone of skin (3 mm in diameter) with a needle and shaving it off with a scalpel. This will result in the removal of around 2 mg of tissue. The tissue is then incubated in normal saline at room temperature for 24 hours to allow the microfilariae (larvae) to emerge. The microfilariae can then be identified microscopically. The sites for the skin snip are usually over the iliac crest, the scapula, and the lower extremities. Six snips provide the most diagnostic sensitivity. Although the skin snip is highly specific, its sensitivity can be limited in the pre-patent stage of infection, which can last 1 to 1.5 years, and in low intensity infections. Performing polymerase chain reaction (PCR) of the skin snip can increase the sensitivity in these two situations, though this is not commercially available.
If a patient has skin nodules caused by onchocercal infection, nodulectomy allows for the identification of macrofilariae (adult worms) in the tissue. Slit lamp eye exam can be used to visualize microfilariae in individuals with eye disease.
There are antibody tests that can assist in the diagnosis of onchocerciasis, though many are not available outside the research setting. There is a general screen for any filarial infection (including Wuchereria, Brugia, Loa, and Mansonella infections) that is available in some specialty diagnostic labs. Because the test is highly sensitive, it is useful in determining if an individual has had filarial infection, but it is not specific enough to identify which filarial infection. As with any antibody test, the results indicate only that the patient has been exposed to the disease, but it cannot determine if the patient has an active infection. This distinction is less important in symptomatic travelers, but it limits the usefulness of the test in persons from endemic areas. One advantage of the test is that it can pick up evidence of infection in the pre-patent stage of infection. There are several Onchocerca-specific serologic tests in existence, such as the OV-16 antigen antibody test and the OV luciferase immunoprecipitation system (LIPS) assay, but these are currently only available in the research setting and are not approved for diagnosis in the United States.
In general the diagnosis of O. volvulus infection should be made with skin snip. However, when skin snips are negative and clinical suspicion of infection is high, the general antibody test could be used in an attempt to exclude infection. If the general antibody test were positive, then it might be necessary to consider performing additional skin snips and/or seeking additional diagnostic information by enlisting the assistance of researchers who perform antibody tests.
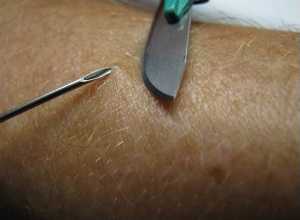
Left: A physician takes a skin sample from a patient for a skin snip biopsy by elevating a piece of skin with a needle and shaving it off with a scalpel.
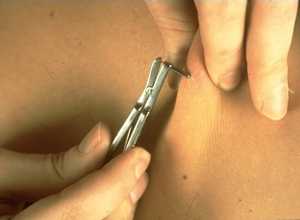
Right: A physician takes a skin sample from a patient for a skin snip biopsy using a sclerocorneal biopsy punch.
Images courtesy of Thomas B. Nutman, MD, Laboratory of Parasitic Diseases, NIAID, NIH
Treatment
General principles of treatment (see table below for dosing and precautions)
The treatment of choice for onchocerciasis is ivermectin, which has been shown to reduce the occurrence of blindness and to reduce the occurrence and severity of skin symptoms. Ivermectin kills the microfilariae (larvae), but not the macrofilariae (adult worms). There is no evidence that prolonged daily treatment provides any benefit over annual treatment, as one dose results in a significant decrease in microfilarial load that lasts a year or more. Treatment with higher than recommended doses has an increased incidence of side effects and may even be harmful. Although ivermectin does not kill the macrofilariae, it does sterilize the females. There is evidence that treating people with ivermectin more frequently than once a year facilitates more rapid sterilization of the female and that treating a person who no longer lives in an endemic area more frequently, such as every 3 to 6 months, could result in a shorter duration of symptoms. Treatment for a patient who will not be returning to live in an endemic area should be given every 6 months (and dosing as frequent as every 3 months could be considered) for as long as there is evidence of continued infection. Evidence of continued infection would include skin symptoms such as pruritus, microfilariae in skin biopsies, and microfilariae on eye exam. Finding adult worms in nodules would not necessarily constitute evidence of the need for continued treatment, as the macrofilariae do not cause symptoms and ivermectin does not kill the macrofilariae. Treatment with ivermectin can cause mild symptoms associated with death of the microfilariae, such as increase itching, but there is no worsening of eye symptoms. Severe adverse reactions to ivermectin in the absence of Loa loa co-infection are rare.
An evolving treatment is doxycycline, which has been shown in studies to kill Wolbachia, an endosymbiotic rickettsia-like bacterium that appears to be required for the survival of the O. volvulus macrofilariae and for embryogenesis. Treatment with a 6-week course of doxycycline has been shown to kill more than 60% of the adult female worms and to sterilize 80 to 90% of the females 20 months after treatment. Doxycycline does not kill the microfilariae, so treatment with ivermectin would be needed to result in a more rapid decrease of symptoms. Most protocols that have examined the effectiveness of doxycycline had given treatment with ivermectin 4 to 6 months after treatment with doxycycline, so the safety of simultaneous treatment is not known. Treating with ivermectin one week prior to starting doxycycline would be reasonable. As doxycycline does not result in the rapid death of the parasites and as most of the mild side effects of ivermectin treatment are thought to be related to rapid release of Wolbachia antigens, the side effect profile of the medication when used for the treatment of onchocerciasis does not appear to be different than that of its use for other indications. There are limited data to suggest that treatment of onchocerciasis with doxycycline in patients co-infected with Loa loa is safe, but this is limited to one randomized controlled trial where only people with Loa microfilarial loads < 8,000 per mL were treated and one community-based study of doxycycline treatment in co-endemic areas in which Loa microfilarial loads were not determined.
Older treatments for onchocerchiasis, such as suramin and diethylcarbamazine should not be used. Suramin has multiple systemic toxicities that limit its use in the presence of other less toxic and effective therapies. Diethylcarbamazine accelerates the development of onchocercal blindness.
Treatments for Onchocerca volvulus
| Usage/Drug | Adult Dose | Pediatric dose |
|---|---|---|
| To kill microfilariae: ivermectin |
150 mcg/kg orally in one dose every 6 months |
150 mcg/kg orally in one dose every 6 months |
| To kill macrofilariae: doxycycline* |
200 mg orally daily for 6 weeks |
200 mg orally daily for 6 weeks |
* Doxycycline is not standard therapy, but several studies support its use and safety. Treatment with ivermectin should be given one week prior to treatment with doxycycline in order to provide symptom relief to the patient. If the patient cannot tolerate 200 mg PO daily of doxycycline, 100mg PO daily is sufficient to sterilize female Onchocerca.
Oral ivermectin is available for human use in the United States.
Doxycycline is available for human use in the United States.
Note on treatment in patients co-infected with Loa loa: Patients with Loa loa co-infection should not be treated for onchocerciasis without consulting an expert on loaiasis due to the risk of a fatal encephalitic reaction to ivermectin. Treatment of co-infected people with doxycycline has only been studied in persons with Loa counts of <8000 microfilariae per mL.
Ivermectin
Note on Treatment in Pregnancy
Ivermectin is pregnancy category C. Data on the use of ivermectin in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated during mass prevention campaigns with ivermectin compared with those who were not. The World Health Organization (WHO) excludes pregnant women from mass prevention campaigns that use ivermectin. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
Note on Treatment During Lactation
Ivermectin is excreted in low concentrations in human milk. Ivermectin should be used in breast-feeding women only when the risk to the infant is outweighed by the risk of disease progress in the mother in the absence of treatment.
Note on Treatment in Pediatric Patients
The safety of ivermectin in children who weigh less than 15kg has not been demonstrated. According to the WHO guidelines for mass prevention campaigns, children who are at least 90 cm tall can be treated safely with ivermectin. The WHO growth standard curves show that this height is reached by 50% of boys by the time they are 28 months old and by 50% of girls by the time they are 30 months old, many children less than 3 years old been safely treated with ivermectin in mass prevention campaigns, albeit at a reduced dose.
Doxycycline
Note on Treatment in Pregnancy
Doxycycline is in pregnancy category D. Doxycycline should not be used in pregnant women due to positive evidence of fetal risk. Doxycycline might be indicated in life-threatening situations where no other treatment is available.
Pregnancy Category D: There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).
Note on Treatment During Lactation
Doxycycline is excreted in breast milk. The American Academy of Pediatrics classifies doxycycline as compatible with breastfeeding, whereas the World Health Organization (WHO) advises to avoid doxycycline in lactating women unless used for malaria. Dental staining and inhibition of bone growth in the infant may occur. Doxycycline should be used during lactation only if the potential benefit of therapy to the mother justifies the known risk to the infant.
Note on Treatment in Pediatric Patients
Doxycycline is contraindicated in children age 8 years and younger as it may cause permanent discoloration of the teeth. The WHO recommends that doxycycline should be used only for life-threatening infections in children age 8 years and younger. Use of doxycycline in children age 8 and younger should be limited to instances when there are contraindications to the use of other appropriate antibiotics and the potential benefit justifies the known risk.
- Page last reviewed: February 19, 2014
- Page last updated: February 19, 2014
- Content source:


 ShareCompartir
ShareCompartir