Acanthamoeba Keratitis Fact Sheet for Healthcare Professionals
The following information is provided as a resource on Acanthamoeba keratitis for physicians and ophthalmologists.
General
Acanthamoeba keratitis, a potentially blinding infection of the cornea, is caused by a free-living protozoan that is ubiquitous in nature, found commonly in water, soil, air, cooling towers, heating, ventilating, and air conditioning (HVAC) systems, and sewage systems.
Acanthamoeba species are classified into three morphologic groups. Group I has large cysts with rounded outer walls (ectocysts) that are clearly separated from the inner walls (endocysts). Group II cysts are smaller, with variable endocyst shapes. Group III cysts are smaller than Group II cysts, with poorly separated walls. The major human pathogens belong to Group II, although A. culbertsoni, from Group III, is also a recognized pathogen 1. Eight Acanthamoeba species have been isolated as etiologic agents in Acanthamoeba keratitis: A. castellanii, A. polyphaga, A. culbertsoni, A. hatchetti, A. rhysodes, A. lugdunensis, A. quina and A. griffini 2-5.
More recent genotyping work has focused on the 18S rRNA gene of Acanthamoeba as a basis for taxonomy of the genus. Twelve lineages referred to as T1-T12 have been identified with the majority of the keratitis causing strains belonging to group T4 6,7.
Acanthamoeba keratitis is a local infection of the eye that does not produce systemic illness. Unlike disseminated Acanthamoeba infection, corneal disease is not associated with immunosuppression. Symptoms of Acanthamoeba keratitis include foreign body sensation, photophobia, decreased visual acuity, tearing, pain and redness of the eye. Diagnosis requires a high index of suspicion, and early diagnosis can greatly improve treatment efficacy. Affected individuals are at risk for permanent visual impairment and blindness.
Epidemiology
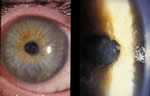
Early epithelial stage of infection. (Photo courtesy of Dan B. Jones, M.D. ) Click here for more images.
Acanthamoeba keratitis primarily affects otherwise healthy people, the majority of whom wear contact lenses. In the United States, an estimated 85% of cases occur in contact lens wearers 8. The incidence of the disease in the United States has been conservatively estimated at approximately one to two cases per million contact lens users, although these estimates need to be refined 9,10. Individuals who exercise proper contact lens-care practices and non-contact lens wearers can also develop the infection. However, individuals who improperly store, handle or disinfect their lenses (e.g. , use tap water or homemade solutions for cleaning), swim/use hot tubs/shower while wearing lenses, come in contact with contaminated water, have minor damage to their corneas, or have previous corneal trauma are at increased risk of infection. No known cases of person-to-person transmission have been reported.
Acanthamoeba likely invade the cornea through a physical opening, such as a minor abrasion, in the corneal epithelium. Contact lens wear may facilitate direct inoculation of Acanthamoeba into the eye and promote infection through mechanical or hypoxic trauma to the cornea. Upon binding to mannose glycoproteins of the corneal epithelium, Acanthamoeba secretes proteins cytolytic to the epithelium as well as proteases that facilitate further penetration 11-15. IgA antibodies normally protect corneal epithelial cells from Acanthamoeba infection; however, certain Acanthamoeba species are capable of producing proteases that lead to antibody degradation 16.
Because the timing of exposure to Acanthamoeba is difficult to assess and because the time required to establish infection is highly dependent on the size of the inoculum, the incubation period for Acanthamoeba keratitis is difficult to determine; it is thought to range from several days to several weeks.
Life Cycle and Morphology
Acanthamoeba exists in two forms: an active, infective trophozoite and a dormant, environmentally hardy cyst. Trophozoites measure approximately 25 to 50 μm in diameter with a single nucleus, dense nucleolus, and filamentous projections called acanthopodia. Trophozoites reproduce by binary fission and feed on a variety of organisms, including cyanobacteria, bacteria, fungi, and other protozoa. In the resilient double-walled cyst form, Acanthamoeba can survive for years under adverse conditions, such as extreme temperatures and pH, 17 desiccation, 18 and chemical exposure 19.
Disease
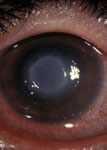
Early inflammation due to Acanthamoeba keratitis. This can resemble keratitis caused by herpes simplex. (Photo courtesy of Dan B. Jones, M.D. ) Click here for more images.
The clinical presentation of Acanthamoeba keratitis varies greatly. Affected individuals may complain of unilateral foreign body sensation, photophobia, decreased visual acuity, tearing, and pain or redness of the eye. Infection involving both eyes can occur. Pain out of proportion to clinical findings is a classic feature of Acanthamoeba keratitis; however, especially early in the disease, lack of pain does not preclude the diagnosis. Because of similarities to the clinical manifestations of viral, fungal, or bacterial corneal infection, individuals may be misdiagnosed and treated with improper antimicrobial or corticosteroid therapy. Such therapy may initially alleviate symptoms and further obscure the clinical picture and diagnosis.
Diagnosis
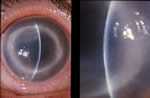
Typical advanced ring infiltrate. (Photo courtesy of Dan B. Jones, M.D. ) Click here for more images.
The first step in diagnosing Acanthamoeba keratitis is to have a high degree of suspicion, especially in a contact lens wearer with a recent diagnosis of another form of keratitis, such as herpes simplex virus keratitis, who is not responding to therapy. Diagnosis is made on the basis of clinical picture and isolation of organisms from corneal culture or detection of trophozoites and/or cysts on histopathology. However, a negative culture does not necessarily rule out Acanthamoeba infection. Confocal microscopy and polymerase chain reaction assays to detect Acanthamoeba may also assist with diagnosis.
Treatment
Early diagnosis is essential for effective treatment of Acanthamoeba keratitis. The infection can be difficult to treat due to the resilient nature of the cyst form. Current treatment regimens usually include a topical cationic antiseptic agent such as polyhexamethylene biguanide (0.02%) or chlorhexidine (0.02%) with or without a diamidine such as propamidine (0.1%) or hexamidine (0.1%). The duration of therapy may last six months to a year. Pain control can be helped by topical cyclopegic solutions and oral nonsteroidal medications. The use of corticosteroids to control inflammation is controversial. Penetrating keratoplasty may help restore visual acuity.
For more information about treatment, see The Medical Letter’s Drugs for Parasitic Infections. [PDF – 1 page]
Prevention
These guidelines should be followed by all contact lens users to reduce the risk of eye infections, including Acanthamoeba keratitis:
- Visit your eye care provider for regular eye examinations.
- Wear and replace contact lenses according to the schedule prescribed by your eye care provider.
- Remove contact lenses before any activity involving contact with water, including showering, using a hot tub, or swimming.
- Wash hands with soap and water and dry before handling contact lenses.
- Clean contact lenses according to instructions from your eye care provider and the manufacturer’s guidelines.
- Never reuse or top off old solution. Use fresh cleaning or disinfecting solution each time lenses are cleaned and stored.
- Never use saline solution or rewetting drops to disinfect lenses. Neither solution is an effective or approved disinfectant.
- Be sure to clean, rub, and rinse your lenses each time you remove your lenses. Rubbing and rinsing your contact lenses will aid in removing harmful microbes and residues.
- Store reusable lenses in the proper storage case.
- Storage cases should be rubbed and rinsed with sterile contact lens solution (never use tap water), emptied, and left open to dry after each use.
- Replace storage cases at least once every three months.
Contact lens users with questions regarding which solutions are best for them should consult their eye care providers. They should also consult their eye care providers if they have any of the following symptoms: eye pain or redness, blurred vision, sensitivity to light, sensation of something in the eye, or excessive tearing.
This information is not meant to be used for self-diagnosis or as a substitute for consultation with a health care provider. If you have any questions about the parasites described above or think that you may have a parasitic infection, consult a health care provider.
References
- Pussard, M. , and Pons, R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica. 1977;13:557-98.
- Centers for Disease Control (CDC). Acanthamoeba keratitis associated with contact lenses–United States. MMWR Morb Mortal Wkly Rep. 1986;35:405-8.
- Yu HS, Kong HH, Kim SY, Hahn YH, Hahn TW, Chung DI. Laboratory investigation of Acanthamoeba lugdunensis from patients with keratitis. Invest Ophthalmol Vis Sci. 2004;45:1418-26.
- Simitzis-Le Flohic AM, Hasle DP, Paniagua-Crespo E, Colin J, Lagoutte F, Donval A, Bellon C. Acanthamoeba keratitis. Epidemiologic and parasitologic study. J Fr Ophtalmol. 1989;12:361-6.
- Ledee DR, Hay J, Byers TJ, Seal DV, Kirkness CM. Acanthamoeba griffini. Molecular characterization of a new corneal pathogen. Invest Ophthalmol Vis Sci. 1996;37:544-50.
- Stothard, D.R. , Schroeder-Diedrich, J.M. , Awwad, M.H. , Gast, R.J. , Ledee, D.R. , Rodriguez-Zaragoza, S. , Dean, C.L. , Fuerst, P.A. , and Byers, T.J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 1998;45:45-54.
- Walochnik, J. , Haller-Schober, E. , Kolli, H. , Picher, D. , Obwaller, A. , and Aspock, H. Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J. Clin. Microbiol. 2000;38:3932-6.
- Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD. Tandem scanning confocal corneal microscopy in the diagnosis of suspected Acanthamoeba keratitis. Ophthalmology. 2006;113:538-47.
- Schaumberg DA, Snow KK, Dana MR. The epidemic of Acanthamoeba keratitis: where do we stand? Cornea. 1998;17:3-10.
- Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331-6.
- Jaison PL, Cao Z, Panjwani N. Binding of Acanthamoeba to [corrected] mannose-glycoproteins of corneal epithelium: effect of injury. Curr Eye Res. 1998;17:770-6. Erratum in: Curr Eye Res 1998;17:1036.
- Morton LD, McLaughlin GL, Whiteley HE. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro.Infect Immun. 1991;59:3819-22.
- Yang Z, Cao Z, Panjwani N. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect Immun. 1997;65:439-45.
- Hurt M, Niederkorn J, Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3424-31.
- Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun. 1998;66:5-10.
- Leher HF, Alizadeh H, Taylor WM, Shea AS, Silvany RS, Van Klink F, Jager MJ, Niederkorn JY. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 1998;39:2666-73.
- Biddick CJ, Rogers LH, Brown TJ. Viability of pathogenic and nonpathogenic free-living amoebae in long-term storage at a range of temperatures. Appl Environ Microbiol. 1984;48:859-60.
- Neff RJ, Neff RH. The biochemistry of amoebic encystment. Symp Soc Exp Biol. 1969;23:51-81.
- Brandt FH, Ware DA, Visvesvara GS. Viability of Acanthamoeba cysts in ophthalmic solutions. Appl Environ Microbiol. 1989;55:1144-6.
- Page last reviewed: November 2, 2010
- Page last updated: April 20, 2011
- Content source:


 ShareCompartir
ShareCompartir