RADIATION DISPERSAL FROM JAPAN
NOTE: This page is archived for historical purposes and is no longer being maintained or updated.
Radiation Basics
What is radiation?
Radiation is energy that travels through space and may be able to penetrate various materials. Visible light, radio waves, and microwaves are types of radiation that are called nonionizing. The kind of radiation that a nuclear power plant produces is called ionizing radiation because it can produce charged particles in matter.
Ionizing radiation is produced by unstable atoms. Unstable atoms differ from stable atoms because unstable atoms have an excess of energy or mass or both. Radiation can also be produced by high-voltage devices, such as x-ray machines.
Unstable atoms are said to be radioactive. In order to reach stability, these atoms give off, or emit, the excess energy or mass. These emissions are called radiation. The kinds of radiation are electromagnetic (like light) and particulates. Gamma radiation and x rays are examples of electromagnetic radiation. Alpha and beta and alpha radiation are examples of particulate radiation.
What is radioactivity?
Radioactivity is the spontaneous emission of radiation from the nucleus of an unstable atom called an isotope. http://www.ncrponline.org/Publications/138press.html.
What is background radiation?
Background radiation is emitted from radioactive materials in the environment. Almost all background radiation is emitted from materials that occur naturally, and is sometimes referred to as natural background. Some sources of background radiation are radioactive materials that are present in rock and soil, while other sources are produced in the atmosphere by interaction of radiation from space with atoms in the atmosphere. http://hps.org/documents/environmental_radiation_fact_sheet.pdf.
What is an alpha particle?
An alpha particle, which is composed of a nucleus of helium atoms, is a type of radiation that has a very short range (few centimeters in air) and is easily shielded by a single sheet of paper. Alpha particles cannot penetrate the outer layers of skin and are not an external radiation hazard. However, they can be hazardous to your health if ingested or inhaled. http://www.ncrponline.org/Publications/138press.html.
What is a beta particle?
A beta particle, most commonly composed of electrons, has a longer range than an alpha particle (up to several feet) and is less easily shielded. It can cause severe burns with a large amount of exposure, however radiation suits can protect workers from burns. Aluminum foil, plastic, or glass will stop beta particles. Beta particles can penetrate the outer layer of skin and are both an internal and external health hazard. http://www.ncrponline.org/Publications/138press.html.
What is gamma radiation?
Gamma radiation has a very long range and is difficult to shield. Unlike alpha or beta particles, gamma rays are electromagnetic energy waves similar to x rays. Concrete, lead, or steel is needed to shield sources of gamma rays. Gamma radiation can penetrate through the whole body and is an external and internal health hazard. http://www.ncrponline.org/Publications/138press.html.
What is the difference between an external radiation hazard and internal radiation hazard?
An external radiation hazard can result when a source of radiation, for example, a quantity of radioactive material or an x-ray machine, is outside of the body. An internal radiation hazard can result from the deposition of radioactive atoms inside the human body. This can occur as a result of a person breathing radioactive material present as a dust, vapor, or gas; through the ingestion of radioactive materials either in solid or liquid form; through the intake of radioactive materials through wounds; or through absorption of radioactive materials through skin. http://www.ncrponline.org/Publications/138press.html.
What is contamination?
Contamination is the deposition and/or absorption of radioactive material, biological or chemical agents, or hazardous materials on structures, areas, people, or objects. Decontamination is the removal of those materials. Decontamination procedures are straightforward; removing clothing and washing the body thoroughly with soap and water will eliminate most external contamination. http://emergency.cdc.gov/radiation/contamination.asp.
How is radiation measured?
There are several ways to measure radiation. Ground surveys and aerial measurements can be made to determine the extent of contamination on the ground. Air monitoring stations can detect contamination in the air. Soil, vegetation, and crop samples can be analyzed using radiochemical methods to determine the type and amount of radiation present. http://www.ncrponline.org/Publications/138press.html.
Radiation Measurement Systems
Units of Measure
Most scientists in the international community measure radiation using the System Internationale (SI), a uniform system of weights and measures that evolved from the metric system. In the United States, however, the conventional system of measurement is still widely used.
Different units of measure are used depending on what aspect of radiation is being measured. For example, the amount of radiation being given off, or emitted, by a radioactive material is measured using the conventional unit curie (Ci), named for the famed scientist Marie Curie, or the SI unit becquerel (Bq). The radiation dose absorbed by a person (that is, the amount of energy deposited in human tissue by radiation) is measured using the conventional unit rad or the SI unit gray (Gy). The biological risk of exposure to radiation is measured using the conventional unit rem or the SI unit sievert (Sv).
Source: http://emergency.cdc.gov/radiation/measurement.asp
| Units of Measurement | |||
|---|---|---|---|
| Measurements | Explanation | Conventional System (U.S.) | International System (SI) |
| Biological Risk | Risk someone will suffer health effects from radiation exposure | Rem 100 rem= 1 Sv |
Sievert (Sv) |
| Absorbed Dose | Amount of energy deposited per unit of weight of human tissue | Rad 100 rad= 1 Gy |
Gray (Gy) |
| Emitted Radiation | Amount of radiation emitted by radiation source (by number disintegrations per unit of time) | Curie (Ci) 1 uCi= 37,000 Bq |
Becquerel (Bq) |
Source: CDC Radiation Emergencies Fact Sheet (http://www.bt.cdc.gov/radiation/pdf/measurement.pdf)
For additional information about measuring radiation please go to http://emergency.cdc.gov/radiation/measurement.asp
What are the routes of exposure?
An individual can receive a radiation dose from an external source, by loose radioactive material deposited on the skin, or by ingesting or inhaling radiological material. If contamination from the current Japan crisis becomes a concern for workers in the U.S. and its territories, the primary concern would be the inhalation and ingestion of radioactive materials from the fallout. This exposure may cause internal dose to the whole body or to a specific organ.
What factors can affect exposure to radiation?
The amount of radiation released, duration of exposure and distance from the source all affect exposure to radiation. Shielding can help protect the body from external radiation and help limit the exposure to radiation.
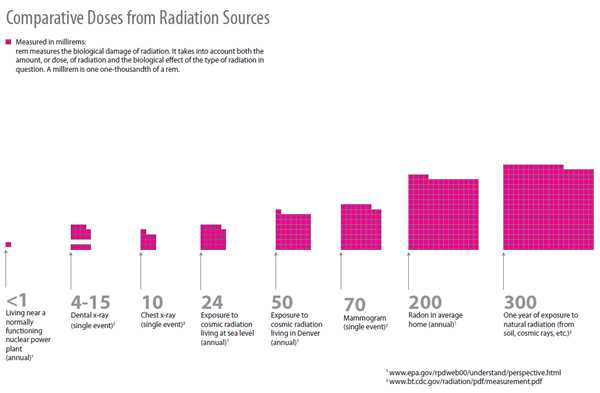
What kinds of radiation is a person exposed to on a normal basis?
For a comparative look at the kinds of radiation a person may experience on a normal, day-to-day and annual basis, please see the chart below.
- Page last reviewed: March 21, 2011 (archived document)
- Content source:
- National Institute for Occupational Safety and Health Office of the Director


 ShareCompartir
ShareCompartir