Division of Bacterial Diseases (DBD) News Bulletin
Winter 2015

Photo: DBD’s Debra Kuehl, associate director for laboratory science, Jennifer Farrar, epidemiologist, and Brian Raphael, Legionella Laboratory lead, help set up a Photoshare World Pneumonia Day Exhibit at CDC’s Atlanta campus on November 9, 2015.
In This Issue
November 12th was World Pneumonia Day

Photo: Jennifer Farrar (right), DBD epidemiologist, collaborating with Shampa Saha, senior research investigator at Bangladesh’s Dhaka Shishu Hospital, on a study evaluating the impact of PCV.
Nearly one million children younger than five years of age die each year from pneumonia. As the most common infectious cause of death in this age group, pneumonia is responsible for 15% of all deaths, with most occurring in resource-limited settings. In developed countries, such as the United States, pneumonia continues to be a leading cause of hospitalization and death, mostly among adults. This infection can be prevented by immunization, adequate nutrition, and addressing environmental factors. Since 2009, people across the globe have annually come together for World Pneumonia Day to raise awareness and promote interventions to protect against, treat, and prevent pneumonia.
CDC is proud to join the Global Coalition against Childhood Pneumonia and our partners around the world in the fight against pneumonia. As the agency’s lead point of contact on pneumonia, DBD engages in activities both globally and domestically to reduce the burden of this disease.
DBD staff provide laboratory and epidemiologic assistance to countries in Africa, Asia, and Latin America that have or plan to introduce pneumococcal conjugate vaccine (PCV) to evaluate vaccine impact and effectiveness. For example, DBD, in collaboration with the Robert Reid Cabral Children’s Hospital, Dominican Republic Ministry of Health, and Pan American Health Organization are evaluating 13-valent PCV (PCV13) against invasive pneumococcal disease (IPD) in the Dominican Republic, which introduced the vaccine into its routine childhood immunization program in 2013. DBD’s ongoing laboratory assessments and trainings for detection and characterization of bacterial respiratory pathogens are helping to strengthen in-country capacity for pneumonia diagnostics at World Health Organization regional laboratories and Ministry of Health national laboratories.
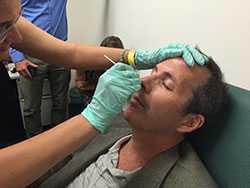
Photo: DBD medical epidemiologist Fernanda Lessa, with Johns Hopkins’ Lee Harrison, demonstrates nasopharyngeal swabbing to staff from Johns Hopkins Bayview Medical Center and the Maryland Active Bacterial Core surveillance site as part of training to support specimen collection for a U.S. adult pneumococcal colonization study, launched in September 2015.
Great strides have been made regarding introduction of vaccines for two main bacterial causes of pneumonia, Haemophilus influenzae type b and Streptococcus pneumoniae. PCV, a highly effective intervention, has dramatically reduced the burden of pneumococcal disease in high-income countries and, with support from DBD staff, is increasingly being used in low- and middle-income countries where the pneumococcal disease burden is the greatest.
The drop in rates of IPD and carriage of vaccine-type S. pneumoniae among U.S. adults over the past decade has been linked to the use of PCV13 in children. In September 2014, the Advisory Committee on Immunization Practices (ACIP) recommended a single dose of PCV13, in addition to the 23-valent pneumococcal polysaccharide vaccine, for adults 65 years or older to prevent cases in this age group. Subsequently, DBD launched studies in the United States on PCV13 impact in adults 65 years or older, including a pneumococcal colonization study, a case-control study with an endpoint of IPD, and an analysis of pneumonia hospitalization trends. Data from these studies, along with information collected using DBD’s Active Bacterial Core surveillance, will be shared with ACIP to help re-evaluate the adult pneumococcal vaccine policy in 2018.
DBD director Rana Hajjeh, notes that, “Over the years, DBD has played an integral role in introducing and evaluating vaccines that can prevent pneumonia, both domestically and globally. These activities are also consistent with CDC’s Global Health Security Agenda, which aims to strengthen capacity for early detection of and response to respiratory disease outbreaks. Along with the Division of Viral Diseases and the Influenza Division, in collaboration with other CDC programs, DBD will develop tools to help countries around the world to detect and respond to respiratory events.”
Director’s Spotlight
Dear colleagues,
Thank you for a very productive year! On behalf of all the OD staff, I wish you restful, safe, and happy holidays with your loved ones.
Regards,
Rana
- Page last reviewed: January 15, 2016
- Page last updated: January 15, 2016
- Content source:


 ShareCompartir
ShareCompartir