Vaccine News, Awards
This website is archived for historical purposes and is no longer being maintained or updated.
January 14, 2014: Content on this page kept for historical reasons.
In This Issue
Division of Bacterial Diseases (DBD) News Bulletin
Fall 2013
Expanded Age Indication for Menveo®
On August 1, 2013, the Food and Drug Administration approved Menveo® for the prevention of meningococcal disease in infants and toddlers from two months of age. This is the first licensed infant meningococcal vaccine that protects against four serogroups of Neisseria meningitidis (A, C, Y, and W-135), which means it can also be used when indicated for travel. The Advisory Committee on Immunization Practices discussed and voted on use of Menveo® for high risk infants at its October meeting.
2012 Data Show More Adolescents are Getting Tdap and MCV4
From 2011 to 2012, vaccination coverage among U.S. adolescents between the ages of 13 and 17 years increased to about 85% for at least one dose of Tdap and 74% for at least one dose of MCV4. Increased Tdap coverage might be due to state school vaccination requirements (40 states required Tdap vaccination for entry into nonresidential middle schools during the 2012–2013 school year), as well as other factors, including providers’ and parents’ awareness that in recent years most states have reported increased cases or outbreaks of pertussis. Increased coverage for meningococcal vaccine might be due partly to state school vaccination requirements; 13 states required MCV4 vaccination for entry into nonresidential middle schools during the 2012–2013 school year.
Expanding Use of a Serogroup B Meningococcal Vaccine
Princeton University is experiencing a prolonged outbreak of serogroup B meningococcal disease, with eight cases reported as of November 29, 2013. CDC, the New Jersey Department of Health, Princeton University officials, and local health authorities have been working closely together since the first case of meningococcal disease associated with Princeton University was reported in March 2013. A serogroup B meningococcal vaccine, which is only licensed for use in Europe and Australia, will be used at Princeton University. FDA has allowed the use of the vaccine at Princeton University under an Investigational New Drug application. Learn more.
Awards and Recognition
 The Pertussis Team in DBD’s Meningitis and Vaccine Preventable Diseases Branch was awarded the “Excellence in Program or Policy Evaluation—Domestic” as part of the CDC and ATSDR Civil Service Honor Awards. The team was awarded this honor for remarkable insight and leading comprehensive efforts to address the 2012 pertussis resurgence in the United States. Thomas Clark (right) accepted the award from CDC Director, Dr. Tom Frieden, on behalf of the team.
The Pertussis Team in DBD’s Meningitis and Vaccine Preventable Diseases Branch was awarded the “Excellence in Program or Policy Evaluation—Domestic” as part of the CDC and ATSDR Civil Service Honor Awards. The team was awarded this honor for remarkable insight and leading comprehensive efforts to address the 2012 pertussis resurgence in the United States. Thomas Clark (right) accepted the award from CDC Director, Dr. Tom Frieden, on behalf of the team.
Anna Acosta (DBD/MVPDB) was awarded the IDWeek 2013 Program Committee Choice Award in recognition of excellence in her abstract submission. This award is given to the four best accepted abstracts overall. The IDWeek Program Committee selected Anna as a recipient based on her outstanding scientific research for the abstract, “Vaccine Effectiveness and Duration of Protection of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis among Adolescents, Washington State, 2012.”
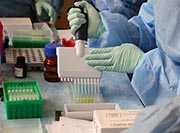 Pamela Cassiday (DBD/MVPDB) won second place in CDC’s International Program category of the annual Public Health in Action Photo Contest. The panel of judges picked the winners based on the quality of the photo and the public health story it tells. Pam’s photo (at left) shows a CDC scientist adding solution to an ELISA plate during pertussis serology training. Since 2010, members of the CDC Pertussis and Diphtheria Laboratory, in conjunction with the Pan American Health Organization and the Sabin Vaccine Institute, have conducted several training courses in Latin America on laboratory diagnosis of pertussis by culture, PCR, and serology.
Pamela Cassiday (DBD/MVPDB) won second place in CDC’s International Program category of the annual Public Health in Action Photo Contest. The panel of judges picked the winners based on the quality of the photo and the public health story it tells. Pam’s photo (at left) shows a CDC scientist adding solution to an ELISA plate during pertussis serology training. Since 2010, members of the CDC Pertussis and Diphtheria Laboratory, in conjunction with the Pan American Health Organization and the Sabin Vaccine Institute, have conducted several training courses in Latin America on laboratory diagnosis of pertussis by culture, PCR, and serology.
Alicia Demirjian (DBD/RDB) and Katherine Fleming-Dutra (DBD/RDB) were awarded the Trainee Award for IDWeek 2013. The Trainee Awards are intended to provide support for fellows-in-training to attend IDWeek. Travel grants will be made available to fellows-in-training in Accreditation Council for Graduate Medical Education-accredited programs in adult and/or pediatric infectious diseases in the United States and Canada, PharmD research training programs in infectious diseases, and other related fellowship programs.
Rita Desai (DBD/MVPDB) successfully completed the requirements of the American Society for Quality (ASQ) to be named an ASQ-Certified Manager of Quality/Organizational Excellence. These important credentials are awarded by the Certification Board of ASQ. This is a formalized recognition that Rita has reached a significant level of professional accomplishment and consistently demonstrates proficiency in and a comprehension of Quality Management principles and practices.
Laurel Garrison (DBD/RDB) was selected as NCIRD’s Employee of the Month for November 2013. This is in recognition of the terrific work she has been doing coordinating Legionnaires’ disease surveillance and outbreaks in the United States and internationally.
Jennifer Whitmon (DBD/MVPDB) recently completed her Doctor of Health Science (D.H.Sc.) degree with a concentration in global health studies from Nova Southeastern University, Florida. The interdisciplinary D.H.Sc. program prepares professionals for application of scientific knowledge to public health, clinical practice, delivery of health services and the education of health professionals. During her internship under the mentorship of Robert Kolter, Harvard professor and past President of the American Society for Microbiology (2010), Jennifer visited industrial and academic microbiological institutions and laboratories in Beijing and Shanghai, China to study the advances in microbiology and their impact on health in the country. Additionally, Jennifer’s practicum project involved creating an educational product for laboratory research professionals in malaria diagnostics development.
- Page last reviewed: January 14, 2014 (archived document)
- Content source:


 ShareCompartir
ShareCompartir