 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Malaria Surveillance --- United States, 2004Jacek Skarbinski, MD,1,2 M. James Eliades,
MD,1,2 Louise M. Causer,
MBBS,2 Ann M. Barber,2 Sonja Mali,
MPH,2 Phuc Nguyen-Dinh, MD,2 Jacquelin M. Roberts,
MS,2 Monica E. Parise, MD,2 Laurence Slutsker,
MD,2 Robert D. Newman, MD2
Corresponding author: Jacek Skarbinski, MD, National Center for Infectious Diseases, 1600 Clifton Road, NE, MS F-22, Atlanta, GA 30333. Telephone: 770-488-7785; Fax: 770-488-4206; E-mail: jskarbinski@cdc.gov. AbstractProblem/Condition: Malaria in humans is caused by any of four species of intraerythrocytic protozoa of the genus Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, or P. malariae). These parasites are transmitted by the bite of an infective female Anopheles sp. mosquito. The majority of malaria infections in the United States occur among persons who have traveled to areas with ongoing malaria transmission. In the United States, cases can occur through exposure to infected blood products, congenital transmission, or local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers. Period Covered: This report summarizes cases in persons with onset of illness in 2004 and summarizes trends during previous years. Description of System: Malaria cases confirmed by blood film are mandated to be reported to local and state health departments by health-care providers or laboratory staff. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS). Data from NMSS serve as the basis for this report. Results: CDC received reports of 1,324 cases of malaria, including four fatal cases, with an onset of symptoms in 2004 among persons in the United States or one of its territories. This number represents an increase of 3.6% from the 1,278 cases reported for 2003. P. falciparum, P. vivax, P. malariae, and P. ovale were identified in 49.6%, 23.8%, 3.6%, and 2.0% of cases, respectively. Seventeen patients (1.3% of total) were infected by two or more species. The infecting species was unreported or undetermined in 262 (19.8%) cases. Compared with 2003, the number of reported malaria cases acquired in the Americas (n = 173) increased 17.7%, whereas the number of cases acquired in Asia (n = 172) and Africa (n = 809) decreased 2.8% and 3.7%, respectively. Of 775 U.S. civilians who acquired malaria abroad, only 160 (20.6%) reported that they had followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Four patients became infected in the United States; three cases were attributed to congenital transmission and one to laboratory-related mosquitoborne transmission. Four deaths were attributed to malaria, including two caused by P. falciparum, one by P. vivax, and one by a mixed infection with P. falciparum and P. malariae. Interpretation: The 3.6% increase in malaria cases in 2004, compared with 2003, resulted primarily from an increase in the number of cases acquired in the Americas but was offset by a decrease in the number of cases acquired in Africa and Asia. This limited increase might reflect local changes in disease transmission, increased travel to regions in which malaria is endemic, or fluctuations in reporting to state and local health departments. These changes likely reflect expected variation in annual reporting and should not be interpreted as indicating a longer-term trend. In the majority of reported cases, U.S. civilians who acquired infection abroad had not adhered to a chemoprophylaxis regimen that was appropriate for the country in which they acquired malaria. Public Health Actions: Additional investigations were conducted for the four fatal cases and four infections acquired in the United States. Persons traveling to a malarious area should take one of the recommended chemoprophylaxis regimens appropriate for the region of travel and use personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently has a fever or influenza-like symptoms should seek medical care immediately and report their travel history to the clinician; investigation should include a blood-film test for malaria. Malaria infections can be fatal if not diagnosed and treated promptly. Recommendations concerning malaria prevention can be obtained from CDC at http://www.cdc.gov/travel or by calling the Malaria Hotline at telephone 770-488-7788. Recommendations concerning malaria treatment can be obtained at http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm or by calling the Malaria Hotline. IntroductionMalaria in humans is caused by infection with one or more of four species of Plasmodium (i.e., P. falciparum, P. vivax, P. ovale, and P. malariae) that can infect humans. Other Plasmodium species infect animals. The infection is transmitted by the bite of an infective female Anopheles sp. mosquito. Malaria remains a devastating global problem, with an estimated 350--500 million cases occurring annually (1). Forty-nine percent of the world's population lives in areas where malaria is transmitted (e.g., parts of Africa, Asia, the Middle East, Eastern Europe, Central and South America, Hispaniola, and Oceania), and approximately 1 million persons die from malaria each year, 80% of them in sub-Saharan Africa (1). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (2). During the late 1940s, a combination of improved housing and socioeconomic conditions, water management, vector-control efforts, and case management was successful at interrupting malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of resistance to antimalarial drugs. Anopheline mosquitoes remain seasonally present in all states except Hawaii. The majority of reported cases of malaria diagnosed each year in the United States are imported from regions where malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products are also reported in the United States. In addition, a limited number of cases are reported that might have been acquired through local mosquitoborne transmission (3). State and local health departments and CDC investigate malaria cases acquired in the United States, and CDC analyzes data from imported cases to detect trends in acquisition. This information is used to guide malaria prevention recommendations for international travelers. For example, an increase in P. falciparum malaria among U.S. travelers to Africa, an area with increasing chloroquine resistance, prompted CDC to change the recommended chemoprophylaxis regimen from chloroquine to mefloquine in 1990 (4). The signs and symptoms of malaria illness are varied, but the majority of patients have fever. Other common symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should be considered for persons with these symptoms who have traveled to an area with known malaria transmission. Malaria also should be considered in the differential diagnosis of persons who have fever of unknown origin, regardless of their travel history. Untreated P. falciparum infections can rapidly progress to coma, renal failure, pulmonary edema, and death. This report summarizes malaria cases reported to CDC regarding persons with onset of symptoms in 2004. MethodsData SourcesMalaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (5). Although both systems rely on passive reporting, the numbers of reported cases might differ because of differences in collection and transmission of data. A substantial difference in the data collected in these two systems is that NMSS receives more detailed clinical and epidemiologic data regarding each case (e.g., information concerning the area to which the infected person has traveled). This report presents only data regarding cases reported to NMSS. Cases of blood-film--confirmed malaria among civilians and military personnel are identified by health-care providers or laboratories. Each confirmed malaria case is reported to local or state health departments and to CDC on a uniform case-report form that contains clinical, laboratory, and epidemiologic information. CDC staff review all report forms when received and request additional information from the provider or the state, if necessary (e.g., when no recent travel to a malarious country is reported). Reports of other cases are telephoned to CDC directly by health-care providers, usually when they are seeking assistance with diagnosis or treatment. Information regarding cases reported directly to CDC is shared with the relevant state health department. All cases that have been acquired in the United States are investigated, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a database and analyzed annually. DefinitionsThe following definitions are used in this report:
This report also uses terminology derived from the recommendations of the World Health Organization (6). Definitions of the following terms are included for reference:
The early and prompt diagnosis of malaria requires that physicians obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of the patient's peripheral blood should be examined for parasites. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood-film quality and examination by experienced laboratory personnel* (Appendix). Select reference laboratories and health departments have the capacity to perform PCR diagnosis of malaria, although this is generally reserved for cases for which blood-film diagnosis of malaria or species determination is inadequate. ResultsGeneral SurveillanceFor 2004, CDC received 1,324 reports concerning cases of malaria occurring among persons in the United States and its territories, representing a 3.6% increase from the 1,278 cases reported with a date of onset in 2003 (7) (Table 1). In 2004, a total of 775 cases occurred among U.S. civilians and 282 cases among foreign civilians (Table 1). In recent years, cases have increased among U.S. civilians and decreased among foreign-born civilians (Figure 1). Plasmodium SpeciesOf the 1,324 cases reported in 2004, the infecting species of Plasmodium was identified in 1,062 (80.2%) cases. P. falciparum and P. vivax were identified in blood films from 49.5% and 23.8% of infected persons, respectively (Table 2). The number of reported cases of P. falciparum decreased 3.8%, from 682 in 2003 to 656 in 2004, and the number of P. vivax infections increased 7.5%, from 293 to 315. Among 964 cases for which both the region of acquisition and the infecting species were known, 81.1% of infections acquired in Africa were attributed to P. falciparum and 10.3% to P. vivax. The converse was true for infections acquired in the Americas and Asia; 56.1% and 81.3%, respectively, were attributed to P. vivax and 35.3% and 11.1% to P. falciparum. Region of Acquisition and DiagnosisAll but four reported cases (n = 1,320) were imported. Of 1,190 imported cases for which the region of acquisition was known, 809 (68.0%) were acquired in Africa, 172 (14.5%) in Asia, and 173 (14.5%) in the Americas (Table 3). A total of 368 (3.0%) imported cases were acquired in Oceania. West Africa accounted for 598 (73.9%) cases acquired in Africa, and India accounted for 113 (65.7%) cases acquired in Asia. In the Americas, 121 (69.9%) cases were acquired in Central America and the Caribbean, followed by 34 (19.7%) cases in South America and 18 (10.4%) cases in Mexico. Information regarding region of acquisition was missing for 130 (9.8%) imported cases. Compared with 2003, the number of reported malaria cases acquired in the Americas increased 17.7%, and the number of cases acquired in Asia and Africa decreased 2.8% and 3.7%, respectively. In the United States, the six health departments reporting the highest number of malaria cases were New York City (n = 214), California (n = 130), Texas (n = 123), New Jersey (n = 75), Maryland (n = 70), and Georgia (n = 65) (Figure 2). Of these, three health departments (New Jersey, New York City, and Texas) reported an increase in cases compared with 2003, and three (California, Georgia, and Maryland) reported a decrease. Interval Between Arrival and IllnessBoth the interval between date of arrival in the United States and onset of illness and the infecting Plasmodium species were known for 617 (46.7%) of the imported malaria cases (Table 4). Symptoms began before arrival in the United States for 75 (12.2%) persons and after arrival for 542 (87.8%) persons. Clinical malaria occurred <1 month after arrival in 344 (80.9%) of the 425 P. falciparum cases and in 64 (43.0%) of the 149 P. vivax cases (Table 4). Nine (1.5%) of 617 persons became ill >1 year after returning to the United States. Imported Malaria CasesImported Malaria Among U.S. Military Personnel In 2004, a total of 32 cases of imported malaria were reported among U.S. military personnel. These cases were reported by state health departments and might not include all cases reported through malaria surveillance activities conducted by the U.S. Department of Defense. Of the 26 patients for whom information regarding chemoprophylaxis use was available, six (23.1%) were not using any chemoprophylaxis, and two (7.7%) had adhered to an incorrect regimen. Imported Malaria Among Civilians Of 1,057 imported malaria cases reported among civilians, 775 (73.3%) occurred among U.S. residents and 282 (26.7%) among residents of other countries (Table 5). Of the 775 imported malaria cases among U.S. civilians, 548 (70.7%) were acquired in Africa, a decrease of 2.3% compared with 2003. Asia accounted for 91 (11.7%) cases of imported malaria among U.S. civilians, and travel to the Central American and Caribbean regions accounted for 73 (9.4%) cases. Of the 282 imported cases among foreign civilians, 177 (62.8%) were acquired in Africa. Antimalarial Chemoprophylaxis UseChemoprophylaxis Use Among U.S. Civilians Information concerning chemoprophylaxis use and travel area was known for 694 (89.5%) of the 775 U.S. civilians who had imported malaria. Of these 694 persons, 452 (65.1%) had not taken any chemoprophylaxis, and 72 (10.4%) had not taken a CDC-recommended drug for the area visited (8). Only 138 (19.9%) U.S. civilians had taken a CDC-recommended medication (8). Data for the specific drug taken were missing for the remaining 32 (4.6%) travelers. A total of 86 (62.3%) patients on CDC-recommended prophylaxis reported taking mefloquine weekly; 34 (24.6%) had taken doxycycline daily; none had taken atovaquone-proguanil daily; and 11 (8.0%) who had traveled only in areas where chloroquine-resistant malaria has not been documented had taken chloroquine weekly. Information on adherence to the drug regimen for these persons is presented in the following section. Seven patients (5.1%) had taken combinations of drugs that included one or more CDC-recommended drug for the travel region. Of the 72 patients taking a nonrecommended drug, 26 (36.1%) reported taking chloroquine either alone or in combination with another ineffective drug during travel to an area where chloroquine resistance has been documented. Malaria Infection After Recommended Prophylaxis Use A total of 160 patients (including 138 U.S. civilians, 15 persons in the U.S. military, three foreign civilians, and four persons for whom information regarding status was missing) contracted malaria after taking a recommended antimalarial drug for chemoprophylaxis. Of these, 62 (38.8%) reported complete compliance with the regimen, and 73 (45.6%) reported noncompliance; compliance was unknown for the remaining 25 (15.6%). Information regarding infecting species was available for 123 (76.9%) patients who had taken a recommended antimalarial drug and undetermined for the remaining 37. Cases of P. vivax or P. ovale After Recommended Prophylaxis Use. Of the 160 patients who had malaria diagnosed after recommended chemoprophylaxis use, 50 (31.3%) had cases that were caused by P. vivax, and five (3.1%) had cases caused by P. ovale. Of the 55 total cases of P. vivax or P. ovale, 25 (45.5%) occurred >45 days after arrival in the United States. These cases were consistent with relapsing infections and do not indicate primary prophylaxis failures. Information was insufficient because of missing data regarding symptom onset or return date to assess whether 19 cases were relapsing infections. Eleven cases, all caused by P. vivax, occurred <45 days after the patient returned to the United States. Five of the 11 patients were known to be noncompliant with their antimalarial chemoprophylaxis regimen. Four patients reported compliance with an antimalarial chemoprophylaxis regimen; one had traveled to Africa, one to Oceania, one to Asia, and one to South America. Two of these patients reported taking mefloquine, one reported using doxycycline, and one reported using both doxycycline and mefloquine; blood samples for serum drug levels were not available. Possible explanations for these cases include inappropriate dosing, unreported noncompliance, malabsorption of the drug, or emerging parasite resistance. For the remaining two patients, no information was available concerning compliance; the region of acquisition was North America for one patient and South America for the other. Cases of P. falciparum and P. malariae After Recommended Prophylaxis Use. The remaining 105 cases of malaria reported among persons who had taken a recommended antimalarial drug for chemoprophylaxis include 58 cases of P. falciparum, seven of P. malariae, three of mixed infection, and 37 for which the infecting species was unidentified. Of the 58 P. falciparum cases among those who reported taking a recommended antimalarial drug, 54 were acquired in Africa, one in Oceania, one in Asia, one in Central America, and one in South America. In 41 (70.7%) of these 58 cases, noncompliance with antimalarials was reported. In 13 (22.4%) cases, patients reported compliance with antimalarial chemoprophylaxis; 11 of these patients had traveled to Africa, one to South America, and one to Papua New Guinea. Seven had reported taking mefloquine, five doxycycline, and one both atovaquone-proguanil and doxycycline for malaria chemoprophylaxis. Blood samples were not available for the 13 patients who reported compliance with a recommended regimen. Patient compliance was unknown for four persons with P. falciparum who reported taking a recommended antimalarial drug for prophylaxis. Six of the seven P. malariae cases among those who reported taking a recommended antimalarial drug were acquired in Africa. One (14.3%) of these patients reported noncompliance with antimalarials, and four (57.1%) reported compliance with a recommended chemoprophylaxis regimen. Three compliant patients used mefloquine, and one used doxycycline. All four had traveled to Africa; blood samples were not available. Purpose of TravelPurpose of travel to areas in which malaria is endemic was reported for 692 (89.3%) of the 775 U.S. civilians with imported malaria (Table 6). The largest proportion (52.6%) represented persons who had visited friends or relatives in malarious areas; the second and third highest proportions, 10.6% and 8.7%, represented persons who had traveled as missionaries or as tourists, respectively. Malaria During PregnancyA total of 30 cases of malaria were reported among pregnant women in 2004, representing 6.9% of cases among women. Nine (30.0%) of the 30 cases occurred among U.S. civilians; eight women had traveled to Africa and one to Asia; five had traveled to visit friends and relatives. Approximately 10.0% of pregnant women and 23.0% of nonpregnant women reported taking malaria chemoprophylaxis. Birth outcomes were not available for any of these 30 women. Malaria Acquired in the United StatesCongenital Malaria Three cases of congenital malaria were reported in 2004 and are described in the following case reports:
Probable Laboratory-Acquired Mosquitoborne Malaria One case of malaria attributed to laboratory-related transmission was reported in 2004 and is described in the following case report:
Four deaths attributable to malaria were reported in 2004 and are described in the following case reports:
DiscussionA total of 1,324 cases of malaria were reported to CDC for 2004, representing an increase of 3.6% from the 1,278 cases reported for 2003. This change primarily resulted from an increase in cases acquired in the Americas. Since 2000, CDC has routinely contacted state health departments to ask for outstanding malaria case reports from the previous reporting year or for a statement that reporting is complete. The limited increase in the number of cases in 2004, compared with 2003, might reflect increased international travel or changing patterns of travel but is more consistent with expected variation in annual reporting and should not be interpreted as representing a longer-term trend. One reason for conducting malaria surveillance is to monitor for prophylaxis failures that might indicate emergence of drug resistance. However, approximately 80% of imported malaria cases among U.S. civilians occurred among persons who were either not taking prophylaxis or taking nonrecommended prophylaxis for the region to which they were traveling. The majority of patients for whom appropriate prophylaxis was reported and adequate information was available regarding species and onset of symptoms to indicate that the infection was a primary one rather than a relapse either reported noncompliance with recommended regimen or provided insufficient information to determine whether these cases represented problems with adherence while using correct antimalarial chemoprophylaxis, malabsorption of the antimalarial drug, or emerging drug resistance. Among patients who reported compliance with a recommended regimen, serum drug levels were not available. Therefore, differentiating among inaccurate reporting of compliance, malabsorption of the antimalarial drug, and emerging drug resistance is impossible. No conclusive evidence existed to indicate a single national or regional source of infection among this group of patients or the failure of a particular chemoprophylactic regimen. Health-care providers are encouraged to contact CDC rapidly whenever they suspect chemoprophylaxis failure to enable CDC to measure serum drug levels of the antimalarial drugs in question. The four fatal cases of malaria that occurred in the United States in 2004 underscore the importance of taking correct precautions and chemoprophylaxis. An earlier review of deaths attributed to malaria in the United States indicated that failure to take or adhere to recommended antimalarial chemoprophylaxis, to promptly seek medical care for posttravel illness, and to promptly diagnose and treat suspected malaria all contributed to fatal outcomes (9). Of particular note, 17 cases of malaria, three among U.S. civilians, were reported in the Dominican Republic in 2004 from urban areas in Duarte Province and resort areas in La Altagracia Province previously thought to be nonmalarious (10). In response to this outbreak, CDC expanded its recommendations for chloroquine prophylaxis to include the affected areas. This underscores the need for effective domestic surveillance to detect cases of malaria acquired in presumed nonmalarious areas to guide antimalarial chemoprophylaxis recommendations and better protect travelers. As of March 2006, the resort areas of La Altagracia Province, but not the urban areas of Duarte Province, remain included in the list of areas with malaria transmission in the Dominican Republic. The occurrence of 30 cases of malaria among pregnant U.S. civilians is also cause for concern. Malaria during pregnancy among nonimmune women is more likely to result in severe disease or contribute to an adverse outcome than malaria in nonpregnant women; the fetus might be adversely affected as well (11). Pregnant travelers should be counseled to avoid travel to malarious areas. If deferral of travel is impossible, pregnant women should be informed that the risks for malaria outweigh those associated with prophylaxis and that safe chemoprophylaxis regimens are available. Specific guidance for pregnant travelers is available at http://www.cdc.gov/travel/mal_preg_pub.htm. The three cases of congenital malaria highlight the importance of obtaining a complete travel and immigration history from pregnant women, including any febrile illnesses or confirmed episodes of malaria. For women with history of travel to or immigration from an area in which malaria is endemic or with a history of malaria before delivery, clinicians should remain alert to the diagnosis of malaria in the neonate or infant. Malaria blood films should be obtained from such neonates and infants should they become ill. For women with a confirmed diagnosis of malaria during the peripartum or postnatal periods, strong consideration should be given to presumptive treatment of the neonate or infant with an antimalarial appropriate for the mother's infecting species and region of acquisition. Signs and symptoms of malaria are often nonspecific, but fever usually is present. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a history of travel to a malarious area. Clinicians should ask all febrile patients for a travel history, including international visitors, immigrants, refugees, migrant laborers, and international travelers. Prompt treatment of suspected malaria is essential because persons with P. falciparum infection are at risk for experiencing life-threatening complications soon after the onset of illness. Ideally, therapy for malaria should be initiated immediately after the diagnosis has been confirmed by a positive blood film. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, the parasite density, and the patient's clinical status (12). If a diagnosis of malaria is suspected and cannot be confirmed, or if a diagnosis of malaria is confirmed but species determination is not possible, antimalarial treatment should be initiated that is effective against P. falciparum. Resistance of P. falciparum to chloroquine is worldwide, with the exception of a limited number of geographic regions (e.g., Central America). Therefore, therapy for presumed P. falciparum malaria should entail the use of a drug effective against such resistant strains (13). Health-care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 7). Physicians seeking assistance with the diagnosis or treatment of patients with suspected or confirmed malaria should call CDC's National Center for Infectious Diseases, Division of Parasitic Diseases at telephone 770-488-7788 during regular business hours or CDC's Emergency Operations Center at telephone 770-488-7100 during evenings, weekends, and holidays (ask to page person on call for Malaria Branch), or access CDC's Internet site at http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm. These resources are intended for use by health-care providers only. Detailed recommendations for preventing malaria are available to the general public 24 hours a day online at http://www.cdc.gov/travel/diseases.htm/malaria. In addition, CDC biannually publishes recommendations in Health Information for International Travel (commonly referred to as The Yellow Book) (8), which is available for purchase from Elsevier at http://www.elsevierhealth.com or telephone 1-800-545-2522; it is also available and updated more frequently on CDC's Internet site at http://www.cdc.gov/travel. CDC provides assistance for diagnostic parasitology through DPDx, a project developed and maintained by CDC's Division of Parasitic Diseases. DPDx (available at http://www.dpd.cdc.gov/dpdx) provides free Internet-based laboratory diagnostic assistance (i.e., telediagnosis) to laboratorians and pathologists in suspected parasitic disease cases, such as malaria. Digital images captured from diagnostic specimens can be submitted for consultation through electronic mail. Telediagnosis assistance by CDC is available during regular business hours. Because laboratories can transmit images to CDC and obtain a rapid response (average time: minutes to several hours) to their inquiries, this system allows efficient diagnosis of challenging cases and rapid dissemination of information. As of March 2006, approximately 54 public health laboratories in 45 states and Puerto Rico either have or are in the process of acquiring the hardware needed to perform telediagnosis. Implementation of telediagnosis at public health laboratories receives full assistance from CDC, including training of personnel in digital imaging techniques. The DPDx Internet site also contains reference material with images, text, and videos on approximately 100 different species of parasites with information (including laboratory diagnosis, geographic distribution, clinical features, treatment, and life cycles) available for each parasite. Acknowledgments The authors acknowledge the state, territorial, and local health departments; health-care providers; and laboratories for reporting this information to CDC. References
* To obtain confirmation diagnosis of blood films from questionable cases and to obtain appropriate treatment recommendations, contact either your state or local health department or CDC's National Center for Infectious Diseases, Division of Parasitic Diseases, Malaria Branch at 770-488-7788. AppendixMicroscopic Procedures for Diagnosing MalariaTo establish the diagnosis of malaria, a blood film must be prepared from fresh blood obtained by pricking a patient's finger with a sterile, nonreusable lancet (Figure A-1). Two types of blood films can be used: thin films (as used for hematology) and thick films. Thick and thin films can be made as separate or as combination slides (Figure A-2). Thick blood films are more sensitive in detecting malaria parasites because the blood is concentrated, allowing a greater volume of blood to be examined. However, thick films are more difficult to read. The thin film should be air-dried, fixed with methanol, and allowed to dry before staining; the thick film should also be thoroughly dried but stained without fixation. For best staining results, blood films should be stained with a 2.5% Giemsa solution (pH of 7.2) for 45 minutes (alternate: 7.5% Giemsa for 15 minutes). A combined Wright-Giemsa stain can also detect malaria parasites but does not demonstrate Schüffner's dots as reliably as Giemsa. Plasmodium parasites are always intracellular, and they demonstrate, if stained correctly, blue cytoplasm with a red chromatin dot. Common errors in reading malaria films can be caused by platelets overlying a red blood cell, concern regarding missing a positive slide, and misreading artifacts as parasites. In P. falciparum infections, the parasite density should be estimated by counting the percentage of red blood cells infected (not the number of parasites) under an oil immersion lens on a thin film. Persons suspected of having malaria, but whose blood films do not indicate the presence of parasites, should have blood films repeated approximately every 12--24 hours for 3 consecutive days. If films remain negative, then the diagnosis of malaria is unlikely. A useful complement to microscopy may be found in polymerase chain reaction (e.g., when microscopy fails to determine parasite species or for confirming negative blood smears). Additional information regarding, collection and preparation of blood films is available at CDC's Division of Parasitic Diseases Internet site, DPDx --- Laboratory Identification of Parasites of Public Health Concern (http://www.dpd.cdc.gov/DPDx). Figure A-1 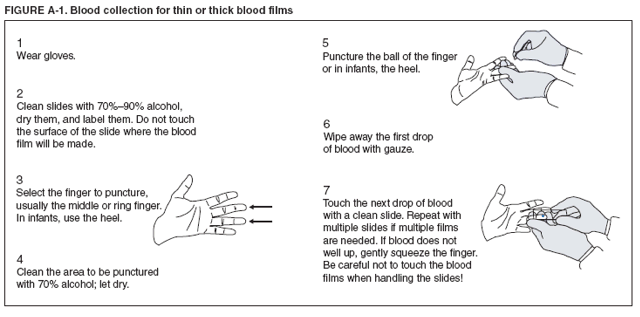 Return to top. Figure A-1 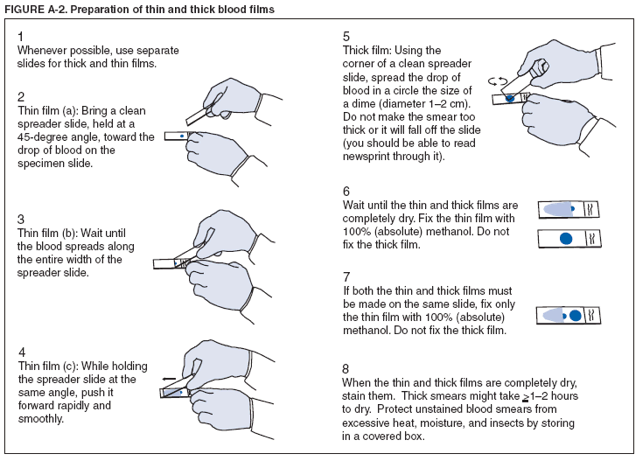 Return to top. Table 1 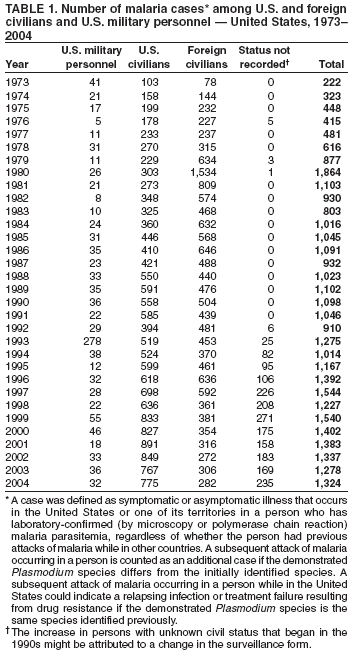 Return to top. Figure 1 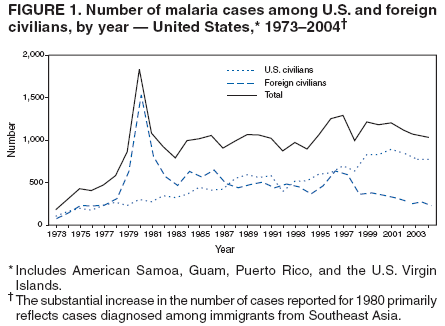 Return to top. Table 2 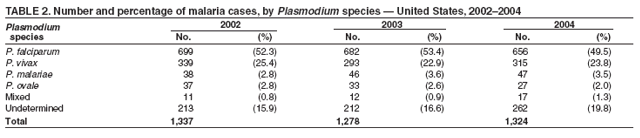 Return to top. Figure 2 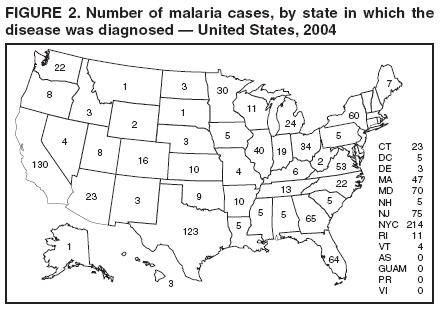 Return to top. Table 3 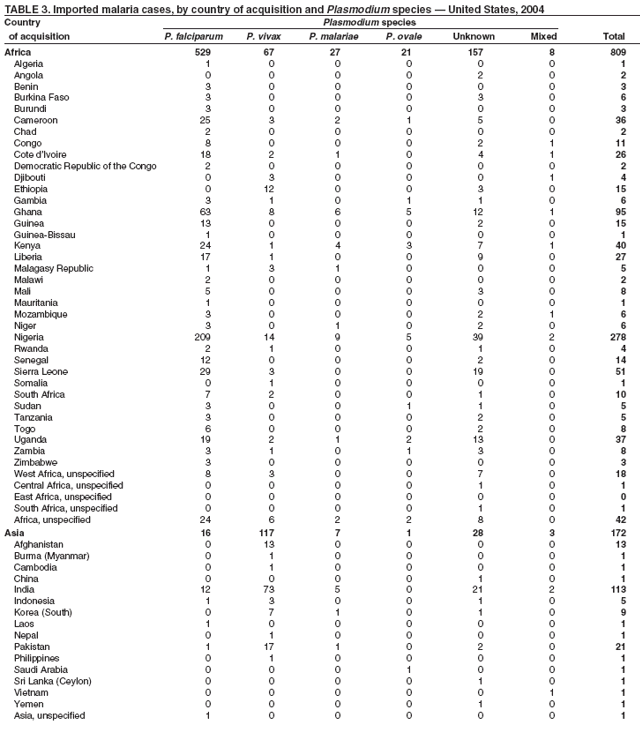 Return to top. Table 4 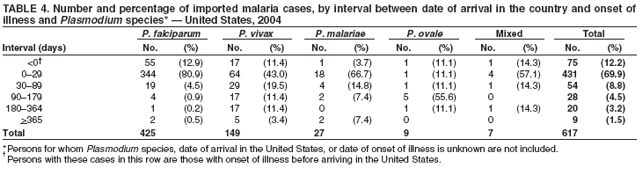 Return to top. Table 5 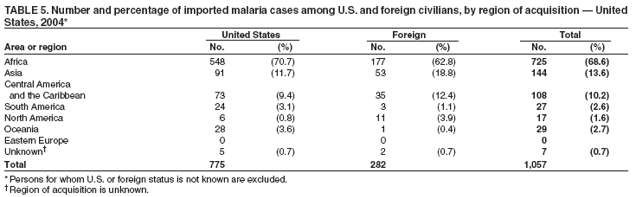 Return to top. Table 6 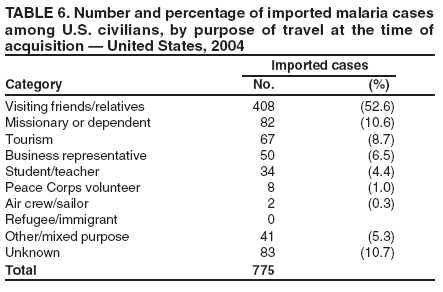 Return to top. Table 7  Return to top.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/2/2006 |
|||||||||
|