 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Influenza --- United States, 1997--98, 1998--99, and 1999--00 SeasonsT. Lynnette Brammer, M.P.H. Abstract Problem/Condition: In the United States, influenza epidemics occur nearly every winter and are responsible for substantial morbidity and mortality, including an average of approximately 114,000 hospitalizations and 20,000 deaths/year. Reporting Period: This report summarizes both actively and passively collected U.S. influenza surveillance data from October 1997 through September 2000. Description of System: During each October--May in the period covered, CDC received weekly reports from 1) approximately 120 World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories in the United States regarding influenza virus isolations; 2) approximately 230, 375, and 430 sentinel physicians during 1997--98, 1998--99, and 1999--00, respectively, regarding their total number of patient visits and the number of visits for influenza-like illness (ILI); and 3) state and territorial epidemiologists regarding estimates of local influenza activity. WHO collaborating laboratories also submitted influenza isolates to CDC for antigenic analysis. Throughout the year, the vital statistics offices in 122 cities reported weekly on deaths related to pneumonia and influenza (P&I). Results: During the 1997--98 influenza season, influenza A(H3N2) was the most frequently isolated influenza virus type/subtype. Influenza A(H1N1) and B viruses were reported infrequently. The proportion of respiratory specimens testing positive for influenza peaked at 28% in late January. The longest period of sustained excess mortality (when the percentage of deaths attributed to P&I exceeded the epidemic threshold) was 10 consecutive weeks. P&I mortality peaked at 9.8% in January. Visits for ILI to sentinel physicians exceeded baseline levels for 7 weeks and peaked at 5% in mid-January through early February. A total of 45 state epidemiologists reported regional or widespread activity at the peak of the season. During the 1998--99 season, influenza A(H3N2) viruses predominated; however, influenza B viruses were also identified throughout the United States. Influenza A(H1N1) viruses were identified rarely. The proportion of respiratory specimens testing positive for influenza peaked at 28% in early February. P&I mortality exceeded the epidemic threshold for 12 consecutive weeks and peaked at 9.7% in early March. Visits for ILI to sentinel physicians exceeded baseline levels for 7 weeks and peaked at 5% in early through mid-February. Forty-three state epidemiologists reported regional or widespread activity at the peak of the season. During the 1999--00 season, influenza A(H3N2) viruses predominated, but influenza A(H1N1) and B viruses also were identified. The proportion of respiratory specimens testing positive for influenza peaked at 31% in mid- to late December. The proportion of deaths attributed to P&I exceeded the epidemic threshold for 13 consecutive weeks and peaked at 11.2% in mid-January. Visits to sentinel physicians for ILI exceeded baseline levels 4 consecutive weeks and peaked at 6% in late December. Forty-four state epidemiologists reported regional or widespread activity at the peak of the season. Interpretation: Influenza A(H1N1), A(H3N2), and B viruses circulated during 1997--2000, but influenza A(H3N2) was the most frequently reported virus type/subtype during all three seasons. Influenza A(H3N2) is the virus type/subtype most frequently associated with excess P&I mortality. Influenza activity during all three seasons occurred at moderate to severe levels, and excess P&I mortality was reported during >10 weeks each year. Public Health Actions: CDC conducts active national surveillance during each October--May to detect the emergence and spread of influenza virus variants and to monitor influenza-related morbidity and mortality. Surveillance data are provided weekly throughout the influenza season to public health officials, WHO, and health-care providers and are used to guide vaccine strain selection, prevention and control activities, and patient care. Influenza vaccination is the most effective means for reducing the yearly effect of influenza. Typically, one or two of the influenza vaccine component viruses are updated each year so that vaccine strains will closely match circulating viruses. Surveillance data will continue to be used to select vaccine strains and to monitor the match between vaccine strains and the currently circulating viruses. Introduction Epidemics of influenza occur nearly every year during the winter months. Influenza is responsible for an average of approximately 114,000 hospitalizations and 20,000 deaths/year in the United States (1,2). Annual vaccination is recommended for groups at increased risk for serious complications from influenza, including adults aged >65 years, persons with certain underlying chronic health conditions (e.g., cardiovascular disease, pulmonary disease, and certain metabolic conditions), and women in their second or third trimester of pregnancy (3). Vaccination of persons in close contact with those at high risk is also recommended. Vaccination is also recommended for all persons aged 50--64 years. Vaccination is recommended for this age group because approximately 24%--32% of persons in this group have high risk conditions that place them at increased risk for influenza-related complications (unpublished data, National Immunization Program, CDC, 2002), and vaccination rates among these persons at high risk have been low. Age-based vaccine recommendations have been more effective than risk-based recommendations for increasing vaccination rates. During influenza epidemics, hospitalization rates among older adults and persons with underlying chronic health problems can increase twofold to fivefold above rates in nonepidemic periods (4). Influenza epidemics are also associated with increased mortality. From 1972--73 through 1994--95, >20,000 influenza-associated deaths/year were estimated to occur during 11 U.S. epidemics, with >40,000 influenza-associated deaths estimated for 6 of these 11 epidemics (5) (unpublished data, National Center for Infectious Diseases, CDC, 1998). During recent years, >90% of influenza-associated deaths have occurred among persons aged >65 years (6). The Advisory Committee on Immunization Practices (ACIP) recommends annual vaccination of persons at high risk for influenza-associated complications as the most effective way to reduce the effects of influenza. ACIP also recommends annual vaccination of persons in frequent contact with persons at high risk to reduce transmission to those at high risk (3). Influenza vaccination is 70%--90% effective in preventing influenza-like illness (ILI) among young, healthy adults when the vaccine antigens closely match the circulating influenza-virus strains (7--10). Elderly persons frequently develop lower postvaccination antibody titers than young, healthy adults and thus can remain susceptible to influenza-related upper respiratory tract infection (11--13). Among elderly persons living outside of nursing homes or similar chronic-care facilities, influenza vaccine is 30%--70% effective in preventing hospitalization for pneumonia and influenza (P&I). Among nursing home residents aged >65 years, influenza vaccine is 30%--40% effective in preventing illness but 80% effective in preventing death (14,15). In addition, vaccination can reduce the risk for outbreaks in nursing home settings (16). Influenza viruses undergo frequent antigenic changes. Because vaccine effectiveness depends on antigenic similarity between vaccine strains and circulating viruses, one or two of the three vaccine component strains typically are updated each year. Both virologic surveillance, in which influenza viruses are isolated for antigenic characterization, and surveillance for related disease activity are necessary to identify influenza virus variants and to determine their ability to spread and cause disease. This information is critical for selecting influenza vaccine strains each year. During October--May, weekly updated summaries of U.S. influenza surveillance data are available from the following sources:
Updated reports regarding seasonal influenza activity also are published multiple times during each influenza season in the MMWR (Weekly). Seasonal influenza activity updates are based on incomplete, preliminary data. This report summarizes influenza activity during the three influenza seasons from October 1997 through September 2000, using the complete and final data sets. MethodsThe sources of influenza surveillance data used during the 1997--98, 1998--99, and 1999--00 seasons were similar to those used in previous years. However, modifications were made to the virologic surveillance, sentinel physician surveillance systems, and the 122 Cities Mortality Reporting System. The effects of influenza vary substantially from year to year. Because influenza is not a nationally notifiable disease and most cases of influenza are not laboratory confirmed, surveillance for influenza is conducted using four methods to monitor influenza virus circulation and influenza's effect on morbidity and mortality. Data from the World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories provide information regarding the virus type/subtypes that are circulating in the United States and each of the nine surveillance regions. These laboratories also provide virus samples used for vaccine strain selection and monitor the match between circulating strains and vaccine components. Data from the sentinel physician system provide an early indication of increasing influenza activity and allow tracking of influenza's effect on physician visits. The State and Territorial Epidemiologist Reports provide an overall assessment of influenza activity in each state. These reports are the only state-based measure of influenza activity. Finally, the 122 Cities Mortality Reporting System tracks the effect of influenza on mortality. Used in combination, these four components provide a complete description of the circulation and effects of the influenza virus. WHO and NREVSS Collaborating LaboratoriesEach week during October--May, approximately 70 WHO and approximately 50 NREVSS collaborating laboratories in the United States reported the number of specimens received for respiratory virus testing and the number testing positive for influenza A(H1N1), A(H3N2), A(not subtyped), or influenza B. The WHO laboratories reported their data by age group (<1 year, 1--4 years, 5--24 years, 25--44 years, 45--64 years, >65 years, or unknown). The 1997--98 influenza season was the first in which influenza virus testing results from the National Respiratory and Enteric Virus Surveillance System (NREVSS) laboratories were used. The laboratories participating in viral surveillance are located primarily in state or local health departments, universities, or hospitals. A subset of the isolates from the WHO laboratories was submitted for complete antigenic characterization and testing for resistance to antiviral agents to the WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza at CDC. Although formal weekly reporting by the WHO laboratories is discontinued during summer months, these laboratories can report influenza viruses isolated during the summer to CDC and submit these viruses for antigenic characterization. NREVSS laboratories report surveillance data year-round. 122 Cities Mortality Reporting System1997--98 and 1998--99 Seasons Each week throughout the year, the vital statistics offices for 122 cities reported the total number of death certificates filed and the number of those in which 1) pneumonia was identified as the underlying cause of death or 2) influenza was listed anywhere on the death certificate. These data were used to calculate the proportion of all deaths attributed to P&I. In addition, these data were used in a periodic regression model to produce a seasonal baseline of expected P&I deaths and calculate an epidemic threshold 1.645 standard deviations above the baseline for that time of year. "Excess" P&I mortality occurred at the point at which the observed proportion of P&I deaths exceeded the epidemic threshold. 1999--00 Season For the 1999--00 season, a new case definition for P&I death was used in the 122 Cities Mortality Reporting System. Before this season, a P&I death was considered to be one in which pneumonia was identified as an underlying cause of death or influenza was listed anywhere on the death certificate. For the 1999--00 influenza season, this case definition was changed so that a P&I death was defined as one in which pneumonia or influenza was listed anywhere on the death certificate. The new case definition could potentially increase P&I mortality measurement levels in comparison with previous seasons. In addition, a limited number of cities use International Classification of Disease (ICD) (17) coding of the underlying cause of death to report P&I deaths to the 122 cities system. In 1999, some of these sites converted from ICD version 9 coding to ICD version 10 coding. ICD 10 coding changed the way that the underlying cause of death is defined and reduced the number of deaths attributed to P&I by approximately 60%. During the 1999--00 season, the effect of these two changes on the national estimate of the percentage of P&I deaths was unknown. The baseline and epidemic threshold values projected for the 1999--00 season used the previous P&I definitions. During summer 2000, an analysis of the P&I mortality data indicated that the combined changes had led to an approximate 0.8% upward shift in the 1999--00 mortality estimates that did not represent a true increase in mortality. To adjust for this upward shift in mortality estimates, the 122 cities P&I mortality baseline was retrospectively adjusted upward for the 1999--00 influenza season. For this report, the 1997--98 and 1998--99 season baseline and epidemic threshold values and proportion of P&I deaths were increased by 0.8 percentage points to allow for comparison with the 1999--00 season. Sentinel Physician Surveillance NetworkEach week during October--May, approximately 230, 375, and 430 volunteer sentinel physicians during the 1997--98, 1998--99, and 1999--00 influenza seasons, respectively, regularly reported (i.e., reported >50% of the time) the number of patients they treated each week and the number of these patients who were seen for ILI,* by age group. Before the 1997--98 influenza season, CDC directly enrolled the sentinel physicians. For the 1997--98 season, CDC and the state health departments began a new approach in which states enrolled and coordinated the sentinel physicians. These changes have resulted in a steady increase in the number of sentinel physicians participating in influenza surveillance. Baseline levels of patient visits for ILI ranged from 0% to 3% of all patient visits. Levels >3% usually correlated with increased influenza activity. Physicians also could submit nasal and throat swabs for virus isolation to either their state public health laboratory or a contract laboratory. State and Territorial Epidemiologist ReportsEstimates of state or territorial influenza activity, as assessed by the state or territorial epidemiologist, were reported weekly to CDC during October--May. Levels were reported as either widespread (i.e., outbreaks of ILI or culture-confirmed influenza in counties having a combined population of >50% of the state's population), regional (i.e., outbreaks of ILI or culture-confirmed influenza in counties having a combined population of <50% of the state's population), sporadic (i.e., sporadically occurring cases of ILI or culture-confirmed influenza, with no outbreaks detected), or no activity. Methods of assessing activity varied from state to state. The sentinel physician surveillance network, WHO, and NREVSS collaborating laboratories data were analyzed nationally and by influenza surveillance region (Figure 1). Only the estimates from state and territorial epidemiologists provide state-specific information. Data from the 122 Cities Mortality Reporting System were analyzed nationally only. Results1997--98 SeasonWHO and NREVSS Collaborating Laboratories and Vaccine Strain Selection During the 1997--98 influenza season, the number of isolates reported by WHO and NREVSS collaborating laboratories peaked during the week ending January 31, 1998 (week 4), when 28% of specimens tested for respiratory viruses were positive for influenza. Of the 12,929 influenza isolates reported to CDC from October 4, 1997, through May 23, 1998, a total of 12,827 (99%) were influenza type A, and 102 (1%) were type B (Figure 2). Of the 3,247 influenza A viruses that were subtyped, 3,241 (99.8%) were influenza A(H3N2), and 6 (0.2%) were influenza A(H1N1). Influenza type B viruses were reported during 27 of the 34 weeks and increased in number later in the season. The proportion of influenza virus types and subtypes was similar among all surveillance regions. The West South Central§ region had the largest number of influenza B isolates, 54 (53%) of the 102 influenza B viruses reported in the United States. However, the 54 B viruses accounted for only 3.4% of all of the influenza viruses identified in the West South Central region. Only <2 influenza A(H1N1) isolates were reported in each of the nine surveillance regions. In all regions except the Pacific region,¶ the proportion of specimens testing positive for influenza peaked between the week ending January 24 and the week ending February 14, 1998 (weeks 3--6). The peak in the Pacific region occurred during the week ending January 3, 1998 (week 53). Active surveillance for the 1997--98 season ended on May 23, 1998. After this date, CDC's strain surveillance laboratory received additional influenza A(H3N2) viruses collected during June--September. CDC also received influenza B viruses collected during June and August. The 1997--98 influenza vaccine contained A/Wuhan/359/95-like(H3N2), A/Bayern/07/95-like(H1N1), and B/Beijing/184/93-like viruses. For the A/Wuhan/359/95-like strain, U.S. vaccine manufacturers used the antigenically equivalent strain A/Nanchang/933/95(H3N2) because of its growth properties. For the same reason, manufacturers used A/Johannesburg/82/96(H1N1) for the A/Bayern/07/95-like (H1N1) strain and B/Harbin/07/94 for the B/Beijing/184/93-like strain. Of the 415 influenza A(H3N2) viruses antigenically characterized by CDC, 59 (14%) were similar to A/Wuhan/359/95, and 356 (86%) were more closely related to the antigenically distinct variant A/Sydney/5/97. A/Wuhan/359/95-like viruses predominated at the beginning of the season, but A/Sydney/5/97 was the predominant strain identified during December and through the remainder of the season. Of the eight influenza A(H1N1) viruses characterized, all but one was antigenically related to A/Bayern/7/95. The remaining A(H1N1) virus was similar to A/Beijing/262/95, an antigenically distinct A(H1N1) variant. All 36 of the influenza B viruses characterized were similar to B/Beijing/184/93. On the basis of these and other data, the 1998--99 influenza vaccine was updated to include A/Sydney/5/97 (H3N2) and A/Beijing/262/95 (H1N1) viruses while retaining the B/Beijing/184/93-like virus as the influenza B component. Although A/Bayern/07/95-like viruses are antigenically distinct from A/Beijing/262/95-like viruses, the A/Beijing/262/95 vaccine strain produces high antibody titers that cross-react with A/Bayern/7/95-like viruses. 122 Cities Mortality Reporting System From October 4, 1997, through May 23, 1998, the percentage of deaths attributed to P&I exceeded the epidemic threshold for 13 of the 34 weeks and peaked at 9.8% for the week ending January 24, 1998 (week 3) (Figure 3). P&I deaths exceeded the epidemic threshold for 10 consecutive weeks, from January 3 through March 14, 1998 (weeks 1--10). Sentinel Physicians Surveillance Network Visits to sentinel physicians for ILI exceeded baseline levels (0%--3%) nationally for 7 consecutive weeks, from the week ending January 3, 1997 (week 53), through the week ending February 14, 1998 (week 6) (Figure 4). The percentage of visits for ILI peaked at 5% during the weeks ending January 24, January 31, and February 7, 1998 (weeks 3--5). The highest percentage of patient visits to sentinel physicians for ILI occurred in the West North Central region** (10%) during the week ending February 7, 1997 (week 5), whereas the West South Central region had the highest number of weeks (11) above baseline levels from the week ending December 20, 1997 (week 51), through the week ending February 28, 1998 (week 8). In contrast, the peak percentage of patient visits for ILI in the East South Central region, which occurred during the week ending February 14, 1998 (week 6), did not exceed baseline levels. State and Territorial Epidemiologist Reports State and territorial epidemiologists first reported regional influenza activity for the week ending October 25, 1997 (week 43), and widespread activity for the week ending December 20, 1997 (week 51) (Figure 5). Influenza activity increased steadily during January and peaked during the weeks ending January 31 and February 7, 1998 (weeks 4 and 5), when 45 states reported regional or widespread activity. Widespread activity was last reported for the week ending April 4, 1998 (week 13), and regional influenza activity was last reported for the week ending May 9, 1998 (week 18). Sporadic activity continued to be reported through the week ending May 23 (week 20), the last week for which reports were available. Outbreak Surveillance During summer 1997, influenza A(H3N2) viruses were associated with outbreaks in 1) a nursing home in California during June, 2) a cruise ship on a cruise from New York City to Montreal from late August through September, and 3) a cruise ship on a cruise from Tahiti to Hawaii during October (18,19). CDC also received influenza A(H3N2) isolates that were collected during July and influenza B isolates that were collected during late May through July. During the 1997--98 influenza season, the majority of reported outbreaks occurred among nursing home residents. However, an outbreak at a military base in Hawaii was also reported (20). Influenza A(H3N2) viruses were responsible for multiple outbreaks during summer 1998. Although the majority of the outbreaks occurred in institutional settings, a substantial regional outbreak also occurred among tourists in Alaska and the Yukon Territory during the summer tourist season (21) as well as in Tennessee among family members who vacationed together during August (22). 1998--99 SeasonWHO and NREVSS Collaborating Laboratories and Vaccine Strain Selection From October 10, 1998, through May 22, 1999, WHO and NREVSS collaborating laboratories tested 102,105 respiratory specimens and identified 14,512 influenza isolates. Of these 14,512 isolates, 11,142 (77%) were influenza type A, and 3,370 (23%) were influenza type B (Figure 2). Of the 2,637 influenza A isolates that were subtyped, 2,607 (99%) were identified as influenza A(H3N2), and 30 (1%) were influenza A(H1N1). The number and proportion of respiratory specimens testing positive for influenza increased during December 1998--February 1999 and peaked during the week ending February 13, 1998 (week 6), when 28% of specimens tested for influenza were positive. Influenza activity in the majority of areas of the country peaked at approximately the same time with the percentage of specimens testing positive for influenza peaking in all nine influenza surveillance regions between weeks 5 and 9. Influenza A(H3N2) was the predominant virus isolated in all nine regions. Of the 30 influenza A(H1N1) viruses isolated, 25 (83%) came from the South Atlantic§§ (11) and Pacific (14) regions. Active influenza surveillance for the 1998--99 season ended May 22, 1999. After this date, CDC's strain surveillance laboratory received additional influenza A(H3N2) viruses collected during June--September. CDC also received influenza B isolates from specimens collected during late May and June. The 1998--99 influenza vaccine contained A/Sydney/5/97-like(H3N2), A/Beijing/262/95-like(H1N1), and B/Beijing/184/93-like viruses. For the B/Beijing/184/93-like strain, U.S. vaccine manufacturers used the antigenically equivalent strain B/Harbin/7/94 because of its growth properties. All 498 of the influenza A(H3N2) viruses antigenically characterized by CDC were similar to A/Sydney/5/97. Of the 17 influenza A(H1N1) viruses characterized, 13 were similar to A/Bayern/7/95, whereas the remaining 4 were similar to A/Beijing/262/95. All influenza B viruses were similar to B/Beijing/184/93. A/Sydney/5/97 (H3N2) and A/Beijing/262/95 viruses were retained for the 1999--00 influenza vaccine. The B/Beijing/184/93-like virus used by the U.S. manufacturers was changed to B/Yamanashi/16/98 because it was most similar to the strains circulating globally at that time. 122 Cities Mortality Reporting System For 1998--99, the percentage of deaths attributed to P&I exceeded the epidemic threshold for 18 of the 33 weeks and peaked at 9.7% during the week ending March 13, 1999 (week 10) (Figure 3). The longest duration in which this percentage was above the epidemic threshold was 12 consecutive weeks, from January 30 through April 17, 1999 (weeks 4--15). Sentinel Physicians Surveillance Network The percentage of patient visits to sentinel physicians for ILI exceeded baseline levels (0%--3%) for 7 consecutive weeks, from January 22 through the week ending March 6, 1999 (weeks 3--9) (Figure 4). Patient visits for ILI peaked at 5% during the weeks ending February 6 through February 20, 1999 (weeks 5--7). Patient visits for ILI among the nine surveillance regions all peaked at 5%--7%. However, the number of weeks for which visits to sentinel physicians was above baseline varied from 4 weeks in the West North Central and Mountain regions¶¶ to 10 weeks in the Mid-Atlantic region.*** State and Territorial Epidemiologist Reports During the 1998--99 influenza season, regional activity was reported by 0--2 states each week from the week ending October 10 through the week ending December 5, 1998 (weeks 40--48) (Figure 5). The number of states reporting regional activity increased to four for the week ending December 12, 1997 (week 49), and widespread activity was first reported the week ending January 2, 1999 (week 52). Influenza activity peaked during the week ending February 20, 1998 (week 7), when 43 states reported regional or widespread activity. Both regional and widespread activity were last reported for the week ending May 8, 1998 (week 18). Outbreak Surveillance Outbreaks associated with influenza A(H3N2) viruses were reported throughout the 1998--99 influenza season and into summer 1999. The majority of reported outbreaks occurred among nursing home residents aged >65 years. However, outbreaks also were reported 1) among tourists to Alaska and the Yukon Territory during May--September (23), 2) on a military base in Texas during July, 3) at multiple correctional facilities in Florida during June--August, 4) in a long-term care facility for the mentally disabled in Florida during July, and 5) on a ship sailing along the northeastern seaboard during September (24). 1999--00 SeasonWHO and NREVSS Collaborating Laboratories and Vaccine Strain Selection During the 1999--00 influenza season, the proportion of respiratory specimens testing positive for influenza as reported by WHO and NREVSS collaborating laboratories peaked at 31% during the week ending December 25, 1999 (week 51). Of the 14,678 influenza isolates reported to CDC from the week ending October 9, 1999, through the week ending May 20, 2000, a total of 14,584 (99%) were influenza type A, and 97 (1%) were type B (Figure 2). Influenza A viruses were reported for all weeks during the 1999--00 season except the week ending May 20, 2000 (week 20). Of the 3,868 influenza A isolates subtyped, 3,715 (96%) were influenza A(H3N2), and 153 (4%) were influenza A(H1N1). All except two regions peaked during the weeks ending December 25, 1999, and January 1, 2000 (weeks 51 and 52). The South Atlantic region peaked during the week ending January 22, 2000 (week 3), and the East South Central region peaked during the week ending February 5, 2000 (week 5). The peak percentages of specimens testing positive for influenza ranged from 20% in the East South Central region to 47% in the Mountain region. Active influenza surveillance for the 1999--00 season ended May 20, 2000. After this date, CDC's strain surveillance laboratory received additional influenza A(H1N1) isolates from specimens collected during late May through September. CDC also received influenza A(H3N2) and B isolates collected during June. The 1999--00 influenza vaccine contained A/Sydney/5/97-like (H3N2), A/Beijing/262/95-like (H1N1), and B/Beijing/184/93-like antigens. For the B/Beijing/184/93-like strain, U.S. vaccine manufacturers used the antigenically equivalent strain B/Yamanashi/166/98 because of its growth properties and its antigenic similarity to circulating viruses. All but two of the influenza A(H3N2) viruses antigenically characterized by CDC were similar to A/Sydney/5/97. The remaining influenza A(H3N2) viruses were similar to A/Panama/2007/99. Of the 148 influenza A(H1N1) viruses characterized, 1 (0.7%) was similar to A/Beijing/262/95; 109 (74%) were similar to the drift variant A/New Caledonia/20/99; and 38 (26%) were similar to A/Bayern/7/95. All 47 of the influenza B viruses characterized at CDC were antigenically similar to B/Beijing/184/93. The 2000--01 influenza vaccine was updated to include an A/Moscow/10/99-like (H3N2) virus and A/New Caledonia/20/99 (H1N1), whereas the B component did not change. A/Panama/2007/99 is antigenically equivalent to A/Moscow/10/99 and was the A(H3N2) strain used in vaccine manufactured for the United States. 122 Cities Mortality Reporting System From October 9, 1999, through May 20, 2000, the percentage of deaths attributed to P&I exceeded the epidemic threshold for 15 of the 33 weeks and peaked at 11.2% for the week ending January 22, 2000 (week 3) (Figure 3). P&I deaths exceeded the epidemic threshold for 13 consecutive weeks, from December 25, 1999, through March 18, 2000 (weeks 51--11). Sentinel Physicians Surveillance Network Visits to sentinel physicians for ILI exceeded baseline levels (0%--3%) for 4 consecutive weeks, from the week ending December 25, 1999, through the week ending January 15, 2000 (weeks 51--2) (Figure 4). The percentage of patient visits for ILI peaked at 6% during the week ending January 1, 2000 (week 52). Activity was similar among the influenza surveillance regions. All nine regions peaked between the week ending December 25, 1999, and the week ending January 15, 2000 (weeks 51--2). The highest percentage of patient visits for ILI was 8% in the West South Central and Pacific regions. In contrast, the lowest percentage of patient visits for ILI was 5% in the New England, Mid-Atlantic, West North Central, South Atlantic, and Mountain regions. The Pacific region also had the longest period above baseline levels (7 weeks), whereas the West North Central region had the shortest (2 weeks). State and Territorial Epidemiologist Reports During the 1999--00 influenza season, regional activity was first reported for the week ending October 9, 1999 (week 40), and continued to be reported by one to three states each week through the week ending November 13, 1999 (week 45) (Figure 5). For the week ending November 20 (week 46), the number of states reporting regional activity increased to six. Widespread activity was first reported for the week ending December 18, 1999 (week 50). Influenza activity continued to increase during December and January and peaked during the week ending January 15, 2000 (week 2), when 44 states reported regional and widespread activity. Activity decreased from late January through March, and the last report of widespread activity was for the week ending February 26, 2000 (week 8). Regional activity was last reported for the week ending April 29, 2000 (week 17), after a 4-week period during which no states reported regional nor widespread activity. Outbreak Surveillance Outbreaks associated with influenza A(H3N2) viruses were reported throughout the fall and winter of the 1999--00 influenza season (25). The majority of reported outbreaks occurred among nursing home residents aged >65 years. However, during July 2000, an outbreak of influenza A(H1N1) occurred among the staff and children attending a summer camp in Texas (26). DiscussionInfluenza A(H3N2) viruses predominated during each of the three influenza seasons from October 1997 through May 2000. Since the reappearance of influenza A(H1N1) viruses in the United States in 1978, influenza A(H1N1), A(H3N2), and B viruses have cocirculated. However, from the 1977--78 season through the 1992--93 season, the same virus type/ subtype never predominated during >2 consecutive seasons. During the influenza seasons from 1993--94 through 1999--00, influenza A(H3N2) viruses predominated during 6 of the 7 seasons and included 4 consecutive A(H3N2) predominant seasons from 1996--97 through 1999--00. Even during the 1995--96 season when A(H1N1) viruses predominated nationally, influenza A(H3N2) viruses were more commonly reported than influenza A(H1N1) viruses in three of the nine influenza surveillance regions. During the three influenza seasons from October 1997 through May 2000, not only did influenza A(H3N2) viruses predominate, but the predominant influenza A(H3N2) strain, A/Sydney/5/97, was the same each season. Since their identification during 1968--69, influenza A(H3N2) viruses typically have been associated with increases in P&I mortality, particularly among the elderly. In each of the 3 influenza seasons from 1997--98 through 1999--00, P&I mortality exceeded epidemic threshold values for >10 consecutive weeks (range: 10--13 weeks). The peak proportion of P&I deaths was 9.7% during 1997--98, 9.8% during 1998--99, and 11.2% during the 1999--00 season, compared with epidemic threshold values of 8.2%, 8.3%, and 8.2%, respectively. Although widespread influenza activity typically occurs during the winter in temperate climates, sporadic cases and occasional outbreaks can occur at any time of the year. Influenza viruses were identified during each month from October 1997 through September 2000, and >1 outbreaks were detected each summer. Because influenza can occur at any time of the year, physicians should include influenza in the differential diagnosis of febrile respiratory illness year-round. Multiple rapid diagnostic tests for influenza are available and can be performed in <30 minutes in a physician's office. The rapid influenza tests fall into three categories. The first detects only influenza type A viruses; the second can detect both influenza type A and B viruses but not distinguish between the two; and the third can detect both influenza type A and B viruses and distinguish the virus type. These tests can be useful for the rapid identification of influenza, particularly in the setting of an institutional outbreak. However, these tests should be used along with viral culture, especially during institutional outbreaks and cases that occur during nonpeak months. Viral culture is the gold standard for influenza virus testing and allows for the confirmation of results from rapid tests that differ in their sensitivity and specificity. Viral culture also provides material for antigenic and genetic characterization. These data are important for influenza vaccine strain selection and assessment of the match between currently circulating viruses and vaccine strains. In addition, influenza viruses identified during summer months might provide important information regarding viral strains that might circulate during the following winter. Since the 1999--00 influenza season, methods of influenza surveillance that are similar to those described in this report continue to be used. Before the 2001--02 season, sufficient P&I mortality data had been collected through the 122 Cities Mortality Reporting System, using the new case definition introduced in 1999 to allow a return to the previous statistical model used by CDC to determine P&I mortality baseline and epidemic threshold values for the 2001--02 season. In addition, since the 1999--00 influenza season, the number of participating sentinel physicians has continued to increase. During the 2000--01 season, approximately 550 physicians reported each week, and approximately 700 physicians reported during 2001--02. During each influenza season, activity updates of preliminary data are published multiple times in the MMWR (Weekly). Data summaries for entire influenza seasons are published periodically in MMWR Surveillance Summaries. References
* For this surveillance system, the case definition for ILI is fever >100ºF (>37.8ºC) and cough or sore throat, in the absence of other confirmed diagnoses. For this surveillance system, the case definition for ILI is left to the discretion of state and territorial health departments. § West South Central region --- Arkansas, Louisiana, Oklahoma, and Texas. ¶ Pacific region --- Alaska, California, Hawaii, Oregon, Washington. ** West North Central region --- Iowa, Kansas, Minnesota, Missouri, Nebraska, North Dakota, and South Dakota. East South Central region --- Alabama, Kentucky, Mississippi, and Tennessee. §§ South Atlantic region --- Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, Washington, D.C., and West Virginia. ¶¶ Mountain region --- Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming. *** Mid-Atlantic region --- New Jersey, New York, and Pennsylvania. New England region --- Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont. Figure 1 Return to top. Figure 2 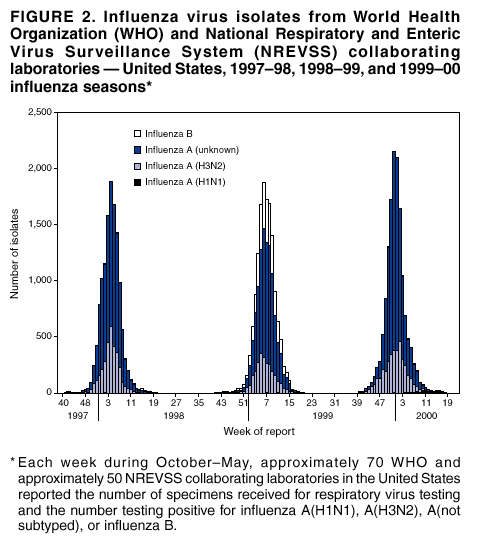 Return to top. Figure 3 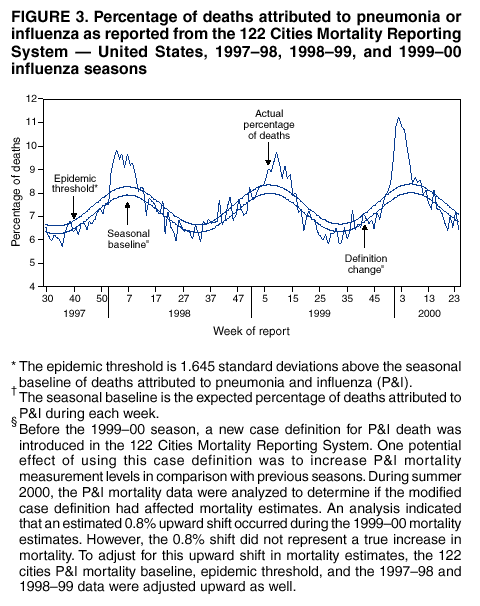 Return to top. Figure 4 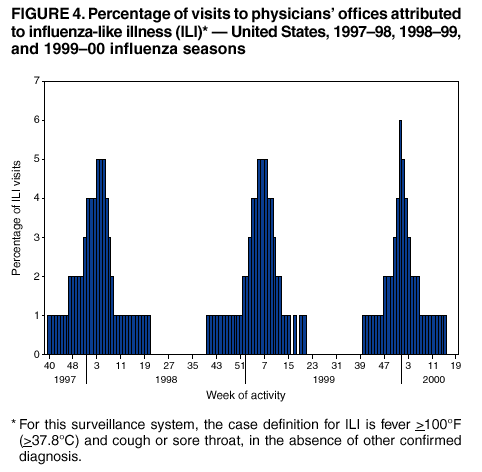 Return to top. Figure 5 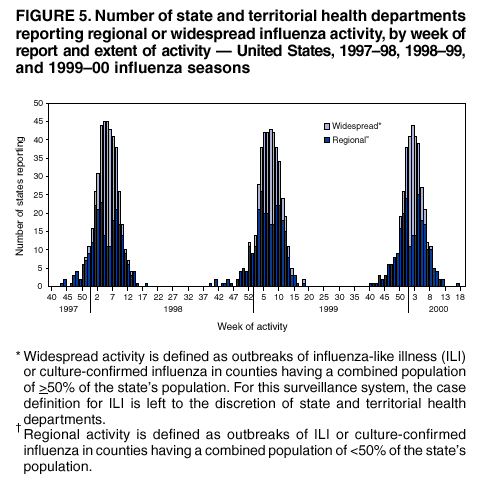 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/9/2002 |
|||||||||
This page last reviewed 10/9/2002
|