Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Use of Influenza A (H1N1) 2009 Monovalent Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009
Summary
This report provides recommendations by CDC's Advisory Committee on Immunization Practices (ACIP) regarding the use of vaccine against infection with novel influenza A (H1N1) virus. Information on vaccination for seasonal influenza has been published previously (CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices [ACIP], 2009. MMWR 2009;58[No. RR-8]). Vaccines against novel influenza A (H1N1) virus infection have not yet been licensed; however, licensed vaccine is expected to be available by mid-October 2009. On July 29, 2009, ACIP reviewed epidemiologic and clinical data to determine which population groups should be targeted initially for vaccination. ACIP also considered the projected vaccine supply likely to be available when vaccine is first available and the expected increase in vaccine availability during the following 6 months. These recommendations are intended to provide vaccination programs and providers with information to assist in planning and to alert providers and the public about target groups comprising an estimated 159 million persons who are recommended to be first to receive influenza A (H1N1) 2009 monovalent vaccine. The guiding principle of these recommendations is to vaccinate as many persons as possible as quickly as possible. Vaccination efforts should begin as soon as vaccine is available. State and local health officials and vaccination providers should make decisions about vaccine administration and distribution in accordance with state and local conditions. Highlights of these recommendations include 1) the identification of five initial target groups for vaccination efforts (pregnant women, persons who live with or provide care for infants aged <6 months, health-care and emergency medical services personnel, children and young adults aged 6 months--24 years, and persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications), 2) establishment of priority for a subset of persons within the initial target groups in the event that initial vaccine availability is unable to meet demand, and 3) guidance on use of vaccine in other adult population groups as vaccine availability increases. Vaccination and health-care providers should be alert to announcements and additional information from state and local health departments and CDC concerning vaccination against novel influenza A (H1N1) virus infection. Additional information is available from state and local health departments and from CDC's influenza website (http://www.cdc.gov/flu).
Introduction
In April 2009, a new influenza A (H1N1) virus, novel influenza A (H1N1) virus, was determined to be the cause of influenza illness in two children in the United States during March and April 2009 (1,2) and the cause of outbreaks of respiratory illness in Mexico (3). This virus was transmitted in communities across North America within weeks and was identified in many areas of the world by May 2009 (4,5). On June 11, 2009, the World Health Organization (WHO) declared a worldwide pandemic, indicating uncontained community-level transmission of the novel influenza A (H1N1) virus in multiple areas of the world (5). Worldwide transmission of the novel influenza A (H1N1) virus has continued since June in both the Northern and Southern Hemispheres (6). Transmission is likely to persist and might increase in the Northern Hemisphere during fall and winter. In contrast to seasonal influenza, current evidence indicates that relatively few severe cases of novel influenza A (H1N1) virus infection have occurred among older persons, and the highest hospitalization rates for illness caused by this virus have been among persons aged <65 years (7). The signs and symptoms of novel influenza A (H1N1) virus infection are similar to those of seasonal influenza, and specific diagnostic testing is required to distinguish novel influenza A (H1N1) virus from seasonal influenza virus (7; CDC, unpublished data, 2009).
Influenza vaccination is the most effective method for preventing influenza and influenza-related complications. However, current seasonal influenza vaccines are not likely to provide protection against novel influenza A (H1N1) virus (8). Specific vaccines against the novel influenza A (H1N1) virus are being manufactured, and licensed vaccine is expected to be available in the United States by mid-October 2009 (9). However, the initial supply of these vaccines might not be enough to meet the demand for vaccine. For this reason, CDC's Advisory Committee on Immunization Practices (ACIP) recommends that certain groups at highest risk for infection or influenza-related complications should be the initial targets for vaccination. Highlights of these recommendations include 1) the identification of five initial target groups for vaccination efforts (pregnant women, persons who live with or provide care for infants aged <6 months, health-care and emergency medical services personnel, children and young adults aged 6 months--24 years, and persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications), 2) establishment of priority for a subset of persons within the initial target groups in the event that initial vaccine availability is unable to meet demand, and 3) guidance on use of vaccine in other adult population groups as vaccine availability increases. Because novel influenza A (H1N1) virus is continuing to cause illness in the United States and worldwide, the primary focus of vaccination efforts should be to vaccinate as many persons as possible in the recommended target groups as quickly as possible once vaccine becomes available. As vaccine availability increases, additional groups are recommended for vaccination. ACIP will review new epidemiologic and clinical data as they become available and might revise these recommendations.
Methods
ACIP provides recommendations to CDC for the prevention and control of vaccine-preventable diseases in the U.S. civilian population. During April--July 2009, the ACIP Influenza Working Group met frequently by teleconference to discuss new information on the spread of novel influenza A (H1N1) virus. In the process of developing vaccination recommendations for consideration by the full ACIP, members considered the evolving burden of illness caused by the virus, the age and risk groups most affected, progress in developing vaccines, anticipated vaccine supply, and various possible vaccination strategies. ACIP's deliberations were informed by consultation with other federal agencies and a review of vaccine allocation guidance developed as part of influenza prepandemic planning during 2007--2008 (10).
The full committee's initial discussions related to novel influenza A (H1N1) virus took place during a public ACIP session held on June 25--26, 2009. At a subsequent public meeting held on July 29, 2009, ACIP made recommendations for use of the influenza A (H1N1) 2009 monovalent vaccine currently in production for the U.S. market. Information presented at these meetings is available at http://www.cdc.gov/vaccines/recs/acip/slides-jun09.htm and http://www.cdc.gov/vaccines/recs/acip/slides-july09-flu.htm.
Background
Human infections with the novel influenza A (H1N1) virus were first identified in April 2009 (1), and infections with this virus have been reported worldwide (5). Because serologic studies suggest that a large majority of the population is susceptible to novel influenza A (H1N1) virus, substantial potential exists for widespread infection (2). The novel influenza A (H1N1) virus is antigenically and genetically distinct from other human influenza A (H1N1) viruses in circulation since 1977 (2). As of August 1, 2009, the novel influenza A (H1N1) viruses circulating worldwide appear to be antigenically similar (11).
Clinical Features
The signs and symptoms of novel influenza A (H1N1) virus infection are similar to those of seasonal influenza (7,12). Definitive diagnosis of novel influenza A (H1N1) virus infection requires specific testing for H1N1 viruses using real-time reverse transcriptase--polymerase chain reaction or viral culture (7,13). Rapid influenza diagnostic tests (RIDTs) for seasonal influenza sometimes can detect novel influenza A (H1N1) virus, but sensitivity has been estimated at 40%--70% (13,14). Negative RIDTs should not be used to exclude the diagnosis of novel influenza A (H1N1) virus infection (13).
The age distribution of confirmed illness, severity of illness, and prevalence of medical risk factors among persons with severe illness have been consistent among many countries and over time. As of July 31, 2009, the median age of persons with laboratory-confirmed infections in the United States was 12 years, and the highest infection incidence was among persons aged 5--24 years (7,11). The incidence of infection was lowest among persons aged ≥65 years. Similar findings have been reported in other countries (15).
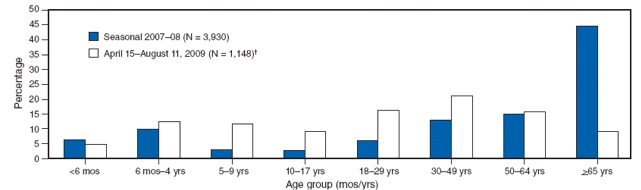
A comparison of the age distribution of hospitalized persons with laboratory-confirmed novel influenza A (H1N1) also demonstrates a striking difference from seasonal influenza (Figure). As of July 31, 2009, the median age of hospitalized persons with laboratory-confirmed novel influenza A (H1N1) virus infection was 20 years, and the incidence of hospitalization was highest among young children aged <4 years (11; CDC, unpublished data, 2009). Only 282 (5%) of 5,514 hospitalizations and 29 (8%) of the 353 reported deaths had occurred among persons aged ≥65 years (CDC, unpublished data, 2009). The median age among persons who died with novel influenza A (H1N1) virus infection was 37 years. In contrast, in multiple studies of seasonal influenza, hospitalization and mortality rates have been highest among persons aged ≥65 years, and an estimated 90% of seasonal influenza-related deaths and 60% of seasonal influenza-related hospitalizations occurred among adults aged ≥65 years (16,17). As of July 31, 2009, only 282 (5%) of 5,514 hospitalizations and 29 (8%) of the 353 reported deaths attributed to novel influenza A (H1N1) virus infection had occurred among persons aged ≥65 years (CDC, unpublished data, 2009). Cumulative novel influenza A (H1N1) hospitalization rates for April--July 2009 approached or exceeded typical end-of-season cumulative rates for seasonal influenza among school-aged children and adults aged 18--49 years in the Emerging Infections Program* (EIP) surveillance areas (11). However, among persons aged ≥65 years, these cumulative hospitalization rates are <20% of the rates typically observed during the winter among persons in this age group. The median age of hospitalized patients during the 2007--08 influenza season in EIP surveillance areas was 59 years, compared with a median age of 26 years for persons hospitalized in these areas during April--July 2009 (CDC, unpublished data, 2009). In addition, outbreaks attributable to novel influenza A (H1N1) viruses among older adults in long-term--care facilities have not been reported even when novel influenza A (H1N1) has been identified among health-care workers in these facilities who worked while ill.
Medical risk factors for severe infection are similar to those identified previously in studies of seasonal influenza (12). In one case series of 179 patients hospitalized with laboratory-confirmed novel influenza A (H1N1) virus infection, 117 (65%) patients had a medical risk factor previously associated with severe infection in studies of seasonal influenza (e.g., chronic heart, lung, renal, liver disease; cancer or immunosuppression; or pregnancy) (12,18; CDC, unpublished data, 2009). Deaths caused by novel influenza A (H1N1) have been reported among pregnant women. In one case series, the incidence of hospitalization for confirmed novel influenza A (H1N1) virus infection among pregnant women was four times higher than that of the general population (19). Obesity (defined as body-mass index [BMI] ≥30) or morbid obesity (BMI ≥40) has been noted among hospitalized patients in some case series (20,21). However, the majority of these patients had other medical risk factors, and investigations to determine whether obesity or morbid obesity is an independent risk factor for severe infection are underway.
Epidemiology and Transmission
The epidemiology of novel influenza A (H1N1) virus infection is under investigation, and epidemiologic characteristics might change as transmission continues. Outbreaks in settings in which young persons congregate (e.g., schools, colleges, and camps) have been a frequent source of community transmission (22,23). During spring and summer 2009, many schools and camps in the United States were dismissed temporarily as a result of outbreak concerns, causing considerable community impact (24).
The number of laboratory-confirmed infections underestimates the incidence of influenza illness caused by novel influenza A (H1N1) virus infection because laboratory testing has been focused on persons with more severe infection. Similar to clinical practice for seasonal influenza, many healthy persons with likely novel influenza A (H1N1) virus infections never are tested because their illness does not require medical intervention or specific diagnosis. Community surveys and population-based telephone surveys in areas with focal outbreaks of novel influenza A (H1N1) virus infection have identified self-reported influenza-like illness (ILI) among approximately 6% of the population in the areas surveyed (CDC, unpublished data, 2009). In June 2009, the New York City Health Department conducted a household survey that indicated that 7% of New Yorkers reported having ILI (fever accompanied by either cough or sore throat) during May 1--20, 2009; because other indicators of ILI (e.g., physician visits for respiratory illness) demonstrated continued and increasing community transmission within New York City, subsequent surveys are likely to indicate that even higher rates of self-reported ILI occurred during late May--June 2009 (25).
Transmission of novel influenza A (H1N1) virus infection in health-care settings has been reported. Among 11 health-care personnel (HCP) with probable or possible patient-to-HCP acquisition and available information on personal protective equipment use, only three HCP reported always using either a surgical mask or an N95 respirator in one case series (26). Acquisition of novel influenza A (H1N1) virus infection by HCP in community settings also has been identified, raising the possibility of introduction of novel influenza A (H1N1) viruses to patients in health-care settings by infected HCP (26).
Vaccination Against Novel Influenza A (H1N1) Virus Infection
Limited data from serologic studies of persons who received vaccination with seasonal influenza vaccines suggest that seasonal influenza vaccines will not provide protection against novel influenza A (H1N1) virus. Among adults, cross-reactive antibody to novel influenza A (H1N1) virus at titers that correlate with protection from illness in studies of seasonal influenza vaccine was detected in 6%--9% of those aged 18--64 years and in 33% of those aged >60 years. No children tested had cross-reactive antibody to novel influenza A (H1N1) virus. Titers of cross-reactive antibody to novel influenza A (H1N1) virus did not increase after administration of seasonal influenza vaccine (2,8).
Vaccines against novel influenza A (H1N1) virus infection are being produced using methods similar to those used for seasonal influenza vaccines. Licensure of vaccines against novel influenza A (H1N1) virus will be based on the same licensure standards used for seasonal influenza vaccines, as is done routinely each year when strains are changed in the seasonal vaccine. Both live, attenuated and inactivated influenza A (H1N1) 2009 monovalent vaccine formulations will be available initially; as with seasonal influenza vaccines, neither of these vaccines will contain adjuvants. The Food and Drug Administration (FDA) and WHO have selected A/California/07/2009 (H1N1) for use as the strain for the vaccines currently being manufactured.
In previously unvaccinated persons aged <9 years, 2 doses of seasonal influenza vaccine are required to induce immunity because young children typically have had limited exposure to influenza viruses and are not immunologically primed (i.e., they do not have preexisting antibodies) (12). The lack of preexisting antibody cross-reactive with the novel influenza A (H1N1) virus among children and younger adults raises the possibility that 2 doses of vaccine (typically separated by ≥21 days) also will be needed to provide protection for persons in these age groups. Ongoing studies will provide additional information about the immune response vaccine, including which groups might need 2 doses. Updated information will be published by CDC in MMWR or will be available at http://www.cdc.gov/flu.
Several vaccines containing an adjuvant also are being studied but probably will not be available initially. These vaccines likely will need to be used under an Emergency Use Authorization.† Additional guidance will be provided if adjuvanted vaccines are made available.
Recommended Use of Influenza A (H1N1) 2009 Monovalent Vaccine
ACIP recommends that vaccination efforts should focus initially on persons in five target groups (Box) whose members are at higher risk for influenza or influenza-related complications, are likely to come in contact with influenza viruses as part of their occupation and could transmit influenza viruses to others in medical care settings, or are close contacts of infants aged <6 months (who are too young to be vaccinated). In the event that vaccine availability is unable to meet initial demand, priority should be given to a subset of the five target groups (Box).
Initial Target Groups
When vaccine is first available, ACIP recommends that programs and providers administer vaccine to persons in the following five target groups (order of target groups does not indicate priority):
- pregnant women,
- persons who live with or provide care for infants aged <6 months (e.g., parents, siblings, and daycare providers),
- health-care and emergency medical services personnel,§
- persons aged 6 months--24 years, and
- persons aged 25--64 years who have medical conditions that put them at higher risk for influenza-related complications.¶
These five target groups comprise an estimated 159 million persons in the United States. This estimate does not accurately account for persons who might be included in more than one category (e.g., a health-care worker with a high-risk condition). Vaccination programs and providers should begin vaccination of persons in all these groups as soon as vaccine is available.
Subset of Target Groups During Limited Vaccine Availability
Current projections of initial vaccine supply indicate that establishment of a subset of the five initial target groups will not be necessary in most areas. However, demand for vaccination and initial supply might vary considerably across geographic areas. If the supply of the vaccine initially available is not adequate to meet demand for vaccination among the five target groups listed above, ACIP recommends that the following subset of the initial target groups receive priority for vaccination until vaccine availability increases (order of target groups does not indicate priority):
- pregnant women,
- persons who live with or provide care for infants aged <6 months (e.g., parents, siblings, and daycare providers),
- health-care and emergency medical services personnel who have direct contact with patients or infectious material,
- children aged 6 months--4 years, and
- children and adolescents aged 5--18 years who have medical conditions that put them at higher risk for influenza-related complications.
This subset of the five target groups comprises approximately 42 million persons in the United States. Vaccination programs and providers should give priority to this subset of the five target groups only if vaccine availability is too limited to initiate vaccination for all persons in the five initial target groups.
Expanding Vaccination Efforts Beyond Initial Target Groups
Decisions about expanding vaccination to include additional populations beyond the five initial target groups should be made at the local level because vaccine availability and demand might vary considerably by area. Once vaccination programs and providers are meeting the demand for vaccine among the persons in the five initial target groups, vaccination should be expanded to all persons aged 25--64 years. Decisions about expanding or establishing priorities for vaccination should be made in accordance with local circumstances based on the judgment of state and local health officials and health-care providers. CDC and other public health agencies will assess the vaccine supply on a continuing basis throughout the manufacturing period. CDC and state and local health authorities will inform providers and the general public if any indication exists of a substantial delay or an inadequate supply.
Current studies indicate the risk for infection among persons aged ≥65 years is less than the risk for persons in younger age groups. Expanding vaccination recommendations to include adults aged ≥65 years is recommended only after assessment of vaccine availability and demand at the local level. Once demand for vaccine among younger age groups is being met, vaccination should be expanded to all persons aged ≥65 years. This recommendation might need to be reassessed as new epidemiologic, immunologic, or clinical trial data warrant and in the context of global need for vaccine.
ACIP makes the following additional recommendations about use of influenza A (H1N1) 2009 monovalent vaccine:
- The number of doses of vaccine required for immunization against novel influenza A (H1N1) has not been established. Because vaccine availability is expected to increase over time, vaccine should not be held in reserve for patients who already have received 1 dose but might require a second dose.
- Simultaneous administration of inactivated vaccines against seasonal and novel influenza A (H1N1) viruses is permissible if different anatomic sites are used. However, simultaneous administration of live, attenuated vaccines against seasonal and novel influenza A (H1N1) virus is not recommended.
- All persons currently recommended for seasonal influenza vaccine, including those aged ≥65 years, should receive the seasonal vaccine as soon as it is available. Recommendations for use of the 2009--10 seasonal influenza vaccine have been published previously (12).
References
- CDC. Swine influenza A (H1N1) infection in two children---Southern California, March--April 2009. MMWR 2009;58:400--2.
- Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009;325:197--201.
- CDC. Outbreak of swine-origin influenza A (H1N1) virus infection---Mexico, March--April 2009. MMWR 2009;58:467--70.
- CDC. Update: novel influenza A (H1N1) virus infections---worldwide, May 6, 2009. MMWR 2009;58:453--8.
- World Health Organization. New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec 2009;84:249--57.
- Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009;324:1557--61.
- Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360:2605--15.
- CDC. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR 2009;58:521--4.
- Robinson R. H1N1 vaccine products and production. In: ACIP presentation slides: special July 2009 meeting [Presentation]. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/vaccines/recs/acip/slides-july09-flu.htm.
- US Department of Health and Human Services, US Department of Homeland Security. Guidance on allocating and targeting pandemic influenza vaccine. Washington, DC: US Department of Health and Human Services, US Department of Homeland Security; 2008. Available at http://www.pandemicflu.gov/vaccine/allocationguidance.pdf.
- CDC. Flu activity and surveillance. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/flu/weekly/fluactivity.htm.
- CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR 2009;58(No. RR-8).
- CDC. Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) virus---United States, 2009. MMWR 2009;58:826--9.
- Faix DJ, Sherman SS, Waterman SH. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 361:728--9.
- Finelli L. Influenza surveillance [Presentation]. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun09/15-2-inf.pdf.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333--40.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179--86.
- Kelly H, Grant K, Williams S, Smith D. H1N1 swine origin influenza infection in the United States and Europe in 2009 may be similar to H1N1 seasonal influenza infection in two Australian states in 2007 and 2008. Influenza Other Respi Viruses 2009;3:183--8.
- Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451--8.
- CDC. Hospitalized patients with novel influenza A (H1N1) virus infection---California, April--May, 2009. MMWR 2009;58:536--41.
- CDC. Intensive-care patients with severe novel influenza A (H1N1) virus infection---Michigan, June 2009. MMWR 2009;58:749--52.
- CDC. Swine-origin influenza A (H1N1) virus infections in a school---New York City, April 2009. MMWR 2009;58:470--2.
- World Health Organization. Preliminary information important for understanding the evolving situation: novel influenza A (H1N1) briefing note 4. Geneva, Switzerland: World Health Organization; 2009. Available at http://www.who.int/csr/disease/swineflu/notes/h1n1_situation_20090724/en/index.html.
- CDC. Technical report for state and local public health officials and school administrators on CDC guidance for school (K--12) responses to influenza during the 2009--2010 school year. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. Available at http://www.cdc.gov/h1n1flu/schools/technicalreport.htm.
- New York City Department of Health and Mental Hygiene. Novel H1N1 influenza update: June 12, 2009. In: New York City Department of Health and Mental Hygiene, Health Alert #22. New York, NY: New York City Department of Health and Mental Hygiene; 2009. Available at http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md22.pdf.
- CDC. Novel influenza A (H1N1) virus infections among health-care personnel---United States, April--May 2009. MMWR 2009;58:641--5.
- CDC. Influenza vaccination of health-care personnel: recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;55(No. RR-2).