 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Prevention and Control of InfluenzaRecommendations of the Advisory Committee on Immunization Practices (ACIP)Please note: An erratum has been published for this article. To view the erratum, please click here. Prepared by
The material in this report originated in the National Center for Immunization and Respiratory Diseases (proposed), Anne Schuchat, MD, Director; Influenza Division (proposed), Nancy Cox, PhD, (Acting) Director; and Immunization Services Division, Lance Rodewald, Director. Corresponding preparer: Joseph Bresee, MD, Influenza Division, National Center for Immunization and Respiratory Diseases, 1600 Clifton Road, N.E., MS A-32, Atlanta, GA 30333. Telephone: 404-639-3747; Fax: 404-639-3866; E-mail: jbresee@cdc.gov. SummaryThis report updates the 2005 recommendations by the Advisory Committee on Immunization Practices (ACIP) regarding the use of influenza vaccine and antiviral agents (CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices [ACIP]. MMWR 2005;54[No. RR-8]:1--44). The 2006 recommendations include new and updated information. Principal changes include 1) recommending vaccination of children aged 24--59 months and their household contacts and out-of-home caregivers against influenza; 2) highlighting the importance of administering 2 doses of influenza vaccine for children aged 6 months--<9 years who were previously unvaccinated; 3) advising health-care providers, those planning organized campaigns, and state and local public health agencies to a) develop plans for expanding outreach and infrastructure to vaccinate more persons than the previous year and b) develop contingency plans for the timing and prioritization of administering influenza vaccine, if the supply of vaccine is delayed and/or reduced; 4) reminding providers that they should routinely offer influenza vaccine to patients throughout the influenza season; 5) recommending that neither amantadine nor rimantadine be used for the treatment or chemoprophylaxis of influenza A in the United States until evidence of susceptibility to these antiviral medications has been re-established among circulating influenza A viruses; and 6) using the 2006--07 trivalent influenza vaccine virus strains: A/New Caledonia/20/1999 (H1N1)-like, A/Wisconsin/67/2005 (H3N2)-like, and B/Malaysia/2506/2004-like antigens. For the A/Wisconsin/67/2005 (H3N2)-like antigen, manufacturers may use the antigenically equivalent A/Hiroshima/52/2005 virus; for the B/Malaysia/2506/2004-like antigen, manufacturers may use the antigenically equivalent B/Ohio/1/2005 virus. A link to this report and other information can be accessed at http://www.cdc.gov/flu. IntroductionIn the United States, epidemics of influenza typically occur during the winter months and have been associated with an average of approximately 36,000 deaths per year in the United States during 1990--1999 (1). Influenza viruses cause disease among all age groups (2--4). Rates of infection are highest among children, but rates of serious illness and death are highest among persons aged >65 years, children aged <2 years, and persons of any age who have medical conditions that place them at increased risk for complications from influenza (2,5--7). Influenza vaccination is the primary method for preventing influenza and its severe complications. As indicated in this report from the Advisory Committee on Immunization Practices (ACIP), annual influenza vaccination is now recommended for the following groups (Box):
Vaccination might prevent hospitalization and death among persons at high risk and might also reduce influenza-related respiratory illnesses and physician visits among all age groups, prevent otitis media among children, and decrease work absenteeism among adults (8--18). Although influenza vaccination levels increased substantially during the 1990s, further improvements in vaccination coverage levels are needed, especially among persons aged <65 years with known risk factors for influenza complications; among blacks and Hispanics aged >65 years; among children aged 6--23 months; and among health-care workers. ACIP recommends using strategies to improve vaccination levels, including using reminder/recall systems and standing orders programs (19--22). Although influenza vaccination remains the cornerstone for the control of influenza, information on antiviral medications also is presented in this report because these agents are an important adjunct to vaccine. Primary Changes and Updates in the RecommendationsThe 2006 recommendations include six principal changes or updates:
Biology of Influenza Influenza A and B are the two types of influenza viruses that cause epidemic human disease (25). Influenza A viruses are further categorized into subtypes on the basis of two surface antigens: hemagglutinin and neuraminidase. Influenza B viruses are not categorized into subtypes. Since 1977, influenza A (H1N1) viruses, influenza A (H3N2) viruses, and influenza B viruses have circulated globally. In 2001, influenza A (H1N2) viruses that probably emerged after genetic reassortment between human A (H1N1) and A (H3N2) viruses began circulating widely. Both influenza A and B viruses are further separated into groups on the basis of antigenic characteristics. New influenza virus variants result from frequent antigenic change (i.e., antigenic drift) resulting from point mutations that occur during viral replication. Influenza B viruses undergo antigenic drift less rapidly than influenza A viruses. Immunity to the surface antigens, particularly the hemagglutinin, reduces the likelihood of infection and severity of disease if infection occurs (26). Antibody against one influenza virus type or subtype confers limited or no protection against another type or subtype of influenza. Furthermore, antibody to one antigenic variant of influenza virus might not completely protect against a new antigenic variant of the same type or subtype (27). Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual incorporation of one or more new strains in each year's influenza vaccine. More dramatic antigenic changes, or shifts, occur less frequently and can result in the emergence of a novel influenza virus with the potential to cause a pandemic. Clinical Signs and Symptoms of Influenza Influenza viruses are spread from person to person, primarily through respiratory droplet transmission (e.g., when an infected person coughs or sneezes in close proximity to an uninfected person) (25). The typical incubation period for influenza is 1--4 days, with an average of 2 days (28). Adults can be infectious from the day before symptoms begin through approximately 5 days after illness onset. Children can be infectious for >10 days after the onset of symptoms, and young children also can shed virus before their illness onset. Severely immunocompromised persons can shed virus for weeks or months (29--32). Uncomplicated influenza illness is characterized by the abrupt onset of constitutional and respiratory signs and symptoms (e.g., fever, myalgia, headache, malaise, nonproductive cough, sore throat, and rhinitis) (33). Among children, otitis media, nausea, and vomiting also are commonly reported with influenza illness (34--36). Uncomplicated influenza illness typically resolves after 3--7 days for the majority of persons, although cough and malaise can persist for >2 weeks. However, among certain persons, influenza can exacerbate underlying medical conditions (e.g., pulmonary or cardiac disease), lead to secondary bacterial pneumonia or primary influenza viral pneumonia, or occur as part of a coinfection with other viral or bacterial pathogens (37). Young children with influenza virus infection can have initial symptoms mimicking bacterial sepsis with high fevers (37,38), and febrile seizures have been reported in up to 20% of children hospitalized with influenza virus infection (35,39). Influenza virus infection also has been uncommonly associated with encephalopathy, transverse myelitis, myositis, myocarditis, pericarditis, and Reye syndrome (35,37,40,41). Respiratory illnesses caused by influenza viruses are difficult to distinguish from illnesses caused by other respiratory pathogens on the basis of signs and symptoms alone (see Role of Laboratory Diagnosis). Reported sensitivities and specificities of clinical definitions of influenza infection that include fever and cough in studies primarily among adults have ranged from 63% to 78% and 55% to 71%, respectively, compared with viral culture (42,43). Sensitivity and predictive value of clinical definitions can vary, depending on the degree of co-circulation of other respiratory pathogens and the level of influenza activity (44). A study of older nonhospitalized patients determined that the presence of fever, cough, and acute onset had a positive predictive value of only 30% for influenza (45), whereas a study of hospitalized older patients with chronic cardiopulmonary disease determined that a combination of fever, cough, and illness of <7 days was 78% sensitive and 73% specific for influenza (46). A study of vaccinated older persons with chronic lung disease indicated that cough was not predictive of influenza virus infection, although having a fever or feverishness was 68% sensitive and 54% specific for influenza virus infection (47). These results highlight the challenges of identifying influenza illness in the absence of laboratory confirmation.
Hospitalizations and Deaths from Influenza The risks for complications, hospitalizations, and deaths from influenza are higher among persons aged >65 years, young children, and persons of any age with certain underlying health conditions (see Persons at Increased Risk for Complications) than among healthy older children and younger adults (1,6,8,48--56). Estimated rates of influenza-associated hospitalizations have varied substantially by age group in studies conducted during different influenza epidemics (Table 1). Among children aged <5 years, hospitalization rates have ranged from approximately 500/100,000 children for those with high-risk medical conditions to 100/100,000 children for those without high-risk medical conditions (57--60). Hospitalization rates among children aged <24 months are comparable to rates reported among persons aged >65 years (59,60) (Table 1). During seasonal influenza epidemics from 1979--80 through 2000--01, the estimated overall number of influenza-associated hospitalizations in the United States ranged from approximately 54,000 to 430,000/epidemic. An average of approximately 226,000 influenza-related excess hospitalizations occurred per year, and 63% of all hospitalizations occurred among persons aged >65 years (61). Since the 1968 influenza A (H3N2) virus pandemic, the number of influenza-associated hospitalizations is generally greater during seasonal influenza epidemics caused by type A (H3N2) viruses than seasons in which other influenza virus types predominate (62). Influenza-related deaths can result from pneumonia and from exacerbations of cardiopulmonary conditions and other chronic diseases. Deaths of adults aged >65 years account for >90% of deaths attributed to pneumonia and influenza (1,54). In one study, approximately 19,000 influenza-associated pulmonary and circulatory deaths per influenza season occurred during 1976--1990, compared with approximately 36,000 deaths during 1990--1999 (1). Estimated rates of influenza-associated pulmonary and circulatory deaths/100,000 persons were 0.4--0.6 among persons aged 0--49 years, 7.5 among persons aged 50--64 years, and 98.3 among persons aged >65 years. In the United States, the number of influenza-associated deaths has increased in part because the number of older persons is increasing, particularly persons aged >85 years (63). In addition, influenza seasons in which influenza A (H3N2) viruses predominate are associated with higher mortality (64); influenza A (H3N2) viruses predominated in 90% of influenza seasons during 1990--1999, compared with 57% of influenza seasons during 1976--1990 (1). Deaths from influenza are uncommon among children both with and without high-risk conditions, but do occur (65,66). A study that modeled influenza-related deaths estimated that an average of 92 deaths (0.4 deaths per 100,000) occurred among children aged <5 years annually during the 1990s, compared with 32,651 deaths (98.3 per 100,000) among adults aged >65 years (1). Of 153 laboratory-confirmed influenza-related pediatric deaths reported from 40 states during the 2003--04 influenza season, 96 (63%) were among children aged <5 years. Sixty-four (70%) of the 92 children aged 2--17 years with influenza who died had no underlying medical condition previously associated with an increased risk for influenza-related complications (67). Options for Controlling InfluenzaIn the United States, the primary option for reducing the effect of influenza is through annual vaccination. Inactivated (i.e., killed virus) influenza vaccines and LAIV are licensed and available for use in the United States (see Recommendations for Using Inactivated and Live, Attenuated Influenza Vaccines). Vaccination coverage can be increased by administering vaccine to persons during hospitalizations or routine health-care visits, as well as at pharmacies, grocery stores, workplaces, or other locations in the community before the influenza season, therefore making special visits to physicians' offices or clinics unnecessary. Achieving increased vaccination rates among persons living in closed settings (e.g., nursing homes and other chronic-care facilities) and among staff can reduce the risk for outbreaks (13), especially when vaccine and circulating strains are well-matched. Vaccination of health-care workers and other persons in close contact with persons at increased risk for severe influenza illness also can reduce transmission of influenza and subsequent influenza-related complications. Antiviral drugs used for chemoprophylaxis or treatment of influenza are adjuncts to vaccine (see Recommendations for Using Antiviral Agents for Influenza) but are not substitutes for annual vaccination. Influenza Vaccine Composition Both the inactivated and live, attenuated vaccines prepared for the 2006--07 season will include A/New Caledonia/20/1999 (H1N1)-like, A/Wisconsin/67/2005 (H3N2)-like, and B/Malaysia/2506/2004-like antigens (for the A/Wisconsin/67/2005 [H3N2]-like antigen, manufacturers may use the antigenically equivalent A/Hiroshima/52/2005 virus, and for the B/ Malaysia/2506/2004-like antigen, manufacturers may use the antigenically equivalent B/Ohio/1/2005 virus). These viruses will be used because they are representative of influenza viruses that are anticipated to circulate in the United States during the 2006--07 influenza season and have favorable growth properties in eggs. Because circulating influenza A (H1N2) viruses are reassortants of influenza A (H1N1) and A (H3N2) viruses, antibodies directed against influenza A (H1N1) and influenza (H3N2) vaccine strains should provide protection against the circulating influenza A (H1N2) viruses. Influenza viruses for both TIV and LAIV are initially grown in embryonated hens eggs, and, therefore, might contain limited amounts of residual egg protein. Therefore, persons with a history of severe hypersensitivity, such as anaphylaxis, to eggs should not receive influenza vaccine. For the inactivated vaccines, the vaccine viruses are made noninfectious (i.e., inactivated or killed) (68). Only subvirion and purified surface antigen preparations of the inactivated vaccine are available. Manufacturing processes vary by manufacturer. Manufacturers might use different compounds to inactivate influenza viruses and add antibiotics to prevent bacterial contamination. Package inserts should be consulted for additional information. Comparison of LAIV with Inactivated Influenza VaccineBoth inactivated influenza vaccine and LAIV are available. Although both types of vaccines are effective, the vaccines differ in several aspects (Table 2). Major Similarities Both LAIV and inactivated influenza vaccines contain strains of influenza viruses that are antigenically equivalent to the annually recommended strains: one influenza A (H3N2) virus, one A (H1N1) virus, and one B virus. Each year, one or more virus strains might be changed on the basis of global surveillance for influenza viruses and the emergence and spread of new strains. Viruses for both vaccines are grown in eggs. Both vaccines are administered annually to provide optimal protection against influenza virus infection (Table 2). Major Differences Inactivated influenza vaccine contains killed viruses, and thus cannot produce signs or symptoms of influenza virus infection. In contrast, LAIV contains live, attenuated viruses and, therefore, has a potential to produce mild signs or symptoms related to influenza virus infection. LAIV is administered intranasally by sprayer, whereas inactivated influenza vaccine is administered intramuscularly by injection. LAIV is more expensive than inactivated influenza vaccine, although the price differential between inactivated vaccine and LAIV has decreased for the 2006--07 season. LAIV is approved only for use among healthy persons aged 5--49 years; inactivated influenza vaccine is approved for use among persons aged >6 months, including those who are healthy and those with chronic medical conditions (Table 2). Efficacy and Effectiveness of Inactivated Influenza Vaccine The effectiveness of inactivated influenza vaccine depends primarily on the age and immunocompetence of the vaccine recipient, the degree of similarity between the viruses in the vaccine and those in circulation, and the outcome being measured. Vaccine efficacy and effectiveness studies might have various endpoints, including the prevention of medically attended acute respiratory illness (MAARI), prevention of culture-positive influenza virus illness, prevention of influenza or pneumonia-associated hospitalizations or deaths, seroconversion to vaccine serotypes, or prevention of seroconversion to circulating influenza virus subtypes. High postvaccination hemagglutination inhibition antibody titers develop in the majority of vaccinated children and young adults (69--71). These antibodies are protective against illness caused by strains that are antigenically similar to those strains of the same type or subtype included in the vaccine (70--73). Children. Children aged >6 months usually acquire protective levels of anti-influenza antibody against specific influenza virus strains after influenza vaccination (69,70,74--79), although the antibody response among children at high risk for influenza-related complications might be lower than among healthy children (80,81). A 2-year randomized study of children aged 6--24 months determined that 89% of children seroconverted to all three vaccine strains during both years (82). During year 1, among 411 children, vaccine efficacy was 66% (95% confidence interval [CI] = 34%--82%) against culture-confirmed influenza (attack rates: 5.5% and 15.9% among vaccine and placebo groups, respectively). During year 2, among 375 children, vaccine efficacy was -7% (CI = -247%--67%; attack rates: 3.6% and 3.3% among vaccine and placebo groups, respectively); the second year exhibited lower attack rates overall and was considered a mild season. In both years of this study, the vaccine strains were well- matched to the circulating influenza virus strains. A randomized study among children aged 1--15 years also demonstrated that inactivated influenza vaccine was 77% and 91% effective against influenza respiratory illness during H3N2 and H1N1 years, respectively (71). One study documented a vaccine efficacy of 56% against influenza illness among healthy children aged 3--9 years (83), and another study determined vaccine efficacy against influenza type B infection and influenza type A infection of 22%--54% and 60%--78% among children with asthma aged 2--6 years and 7--14 years, respectively (84). Two studies have documented that TIV vaccine decreases the incidence of influenza-associated otitis media among young children by approximately 30% (16,17), whereas a third study determined that vaccination did not reduce the burden of acute otitis media (82). Effectiveness of One Dose versus Two Doses of Influenza Vaccine Among Previously Unvaccinated Children Aged <9 Years. Vaccine effectiveness is lower among previously unvaccinated children aged <9 years if they have only received 1 dose of influenza vaccine, compared with children who have received 2 doses. A retrospective study among approximately 5,000 children aged 6--23 months conducted during a year with a suboptimal vaccine match indicated vaccine effectiveness of 49% against medically attended, clinically diagnosed pneumonia or influenza among children who had received 2 doses of influenza vaccine. No effectiveness was demonstrated among children who had received only 1 dose of influenza vaccine, illustrating the importance of administering 2 doses of vaccine to previously unvaccinated children aged <9 years (85). Similar results were observed in a case-control study of children aged 6--59 months with laboratory-confirmed influenza (86). A study assessing protective antibody responses after 1 and 2 doses of vaccine among vaccine-naive children aged 5--8 years also demonstrated the importance of compliance with the 2-dose recommendation (87). When the vaccine antigens do not change from one season to the next, priming with a single dose of vaccine in the spring, followed by a dose in the fall might result in similar antibody responses to a 2-dose regimen in the fall (88,89). Adults Aged <65 Years. When the vaccine and circulating viruses are antigenically similar, influenza vaccine typically prevents influenza illness among approximately 70%--90% of healthy adults aged <65 years (9,12,90,91). Vaccination of healthy adults also has resulted in decreased work absenteeism and decreased use of health-care resources, including use of antibiotics, when the vaccine and circulating viruses are well-matched (9--12,91,92). In a case-control study of adults aged 50--64 years with laboratory-confirmed influenza during the 2003--04 season when the vaccine and circulating viruses were not well-matched, vaccine effectiveness was estimated to be 52% among healthy persons and 38% among those with one or more high-risk conditions (93). Adults Aged >65 Years. An important benefit of the influenza vaccine is its ability to help prevent secondary complications and reduce the risk for influenza-related hospitalization and death among adults aged >65 years with and without high-risk medical conditions (e.g., heart disease and diabetes) (13--15,18,94,95). Older persons and persons with certain chronic diseases might have lower postvaccination antibody titers than healthy young adults and can remain susceptible to influenza virus infection and influenza-related upper respiratory tract illness (96--98). A randomized trial among noninstitutionalized persons aged >60 years reported a vaccine efficacy of 58% against influenza respiratory illness but indicated that efficacy might be lower among those aged >70 years (99). However, among older persons not living in nursing homes or similar chronic-care facilities, influenza vaccine is 30%--70% effective in preventing hospitalization for pneumonia and influenza (15,100). Among older persons who reside in nursing homes, influenza vaccine is most effective in preventing severe illness, secondary complications, and deaths. In this population, the vaccine can be 50%--60% effective in preventing influenza-related hospitalization or pneumonia and 80% effective in preventing influenza-related death, although the effectiveness in preventing influenza illness often ranges from 30% to 40% (101--103). Efficacy and Effectiveness of LAIV The immunogenicity of the approved LAIV has been assessed in multiple studies (104--110), which included approximately 100 children aged 5--17 years and approximately 300 adults aged 18--49 years. LAIV virus strains replicate primarily in nasopharyngeal epithelial cells. The protective mechanisms induced by vaccination with LAIV are not completely understood but appear to involve both serum and nasal secretory antibodies. No single laboratory measurement closely correlates with protective immunity induced by LAIV. Healthy Children. A randomized, double-blind, placebo-controlled trial among 1,602 healthy children initially aged 15--71 months assessed the efficacy of trivalent LAIV against culture-confirmed influenza during two seasons (111,112). This trial included subsets of 238 healthy children (163 vaccinees and 75 placebo recipients) aged 60--71 months who received 2 doses and 74 children (54 vaccinees and 20 placebo recipients) aged 60--71 months who received a single dose during season one, and a subset of 544 children (375 vaccinees and 169 placebo recipients) aged 60--84 months during season two. Children who continued in the study remained in the same study group. In season one, when vaccine and circulating virus strains were well-matched, efficacy was 93% for participants who received 2 doses of LAIV. In season two, when the A (H3N2) component was not well-matched between vaccine and circulating virus strains, efficacy was 86% overall. The vaccine was 92% efficacious in preventing culture-confirmed influenza during the two-season study. Other results included a 27% reduction in febrile otitis media and a 28% reduction in otitis media with concomitant antibiotic use. Receipt of LAIV also resulted in 21% fewer febrile illnesses. A review of LAIV effectiveness in children aged 18 months--18 years found effectiveness against MAARI of 18% but greater estimated efficacy levels: 92% against influenza A (H1N1) and 66% against an influenza B drift variant (113). Healthy Adults. A randomized, double-blind, placebo-controlled trial among 4,561 healthy working adults aged 18--64 years assessed multiple endpoints, including reductions in self-reported respiratory tract illness without laboratory confirmation, absenteeism, health-care visits, and medication use during peak and total influenza outbreak periods (114). The study was conducted during the 1997--98 influenza season, when the vaccine and circulating A (H3N2) strains were not well-matched. During peak outbreak periods, no difference in febrile illnesses between LAIV and placebo recipients was observed. However, vaccination was associated with reductions in severe febrile illnesses of 19% and febrile upper respiratory tract illnesses of 24%. Vaccination also was associated with fewer days of illness, fewer days of work lost, fewer days with health-care--provider visits, and reduced use of prescription antibiotics and over-the-counter medications. Among a subset of 3,637 healthy adults aged 18--49 years, LAIV recipients (n = 2,411) had 26% fewer febrile upper-respiratory illness episodes; 27% fewer lost work days as a result of febrile upper respiratory illness; and 18%--37% fewer days of health-care--provider visits caused by febrile illness, compared with placebo recipients (n = 1,226). Days of antibiotic use were reduced by 41%--45% in this age subset. A randomized, double-blind, placebo-controlled challenge study among 92 healthy adults (LAIV, n = 29; placebo, n = 31; inactivated influenza vaccine, n = 32) aged 18--41 years assessed the efficacy of both LAIV and inactivated vaccine (115). The overall efficacy of LAIV and inactivated influenza vaccine in preventing laboratory-documented influenza from all three influenza strains combined was 85% and 71%, respectively, on the basis of experimental challenge by viruses to which study participants were susceptible before vaccination. The difference in efficacy between the two vaccines was not statistically significant. Cost-Effectiveness of Influenza VaccineInfluenza vaccination can reduce both health-care costs and productivity losses associated with influenza illness. Studies of influenza vaccination of persons aged >65 years conducted in the United States have reported substantial reductions in hospitalizations and deaths and overall societal costs savings (15,100,104). Studies of adults aged <65 years have indicated that vaccination can reduce both direct medical costs and indirect costs from work absenteeism (8,10--12,91,116). Reductions of 13%--44% in health-care--provider visits, 18%--45% in lost workdays, 18%--28% in days working with reduced effectiveness, and 25% in antibiotic use for influenza-associated illnesses have been reported (10,12,117,118). One cost-effectiveness analysis estimated a cost of approximately $60--$4,000/illness averted among healthy persons aged 18--64 years, depending on the cost of vaccination, the influenza attack rate, and vaccine effectiveness against influenza-like illness (ILI) (91). Another cost-benefit economic study estimated an average annual savings of $13.66/person vaccinated (119). In the second study, 78% of all costs prevented were costs from lost work productivity, whereas the first study did not include productivity losses from influenza illness. Economic studies specifically evaluating the cost-effectiveness of vaccinating persons aged 50--64 years are not available, and the number of studies that examine the economics of routinely vaccinating children with TIV or LAIV are limited (8,120--123). However, in a study of inactivated vaccine that included all age groups, cost utility (i.e., cost per year of healthy life gained) improved with increasing age and among those with chronic medical conditions (8). Among persons aged >65 years, vaccination resulted in a net savings per quality-adjusted life year (QALY) gained, whereas among younger age groups, vaccination resulted in costs of $23--$256/QALY. In addition to estimating the economic cost associated with influenza disease, studies have assessed the public's perception of preventing influenza morbidity. Less than half of respondents to a survey on public perception of the value of preventing influenza morbidity reported that they would trade any time from their own life to prevent a case of uncomplicated influenza in a hypothetical child (124). When asked about their willingness to pay to prevent a hypothetical child from having an uncomplicated case of influenza, the median willingness-to-pay amount was $100 for a child aged 14 years and $175 for a child aged 1 year (124). Vaccination Coverage LevelsOne of the national health objectives for 2010 is to achieve an influenza vaccination coverage level of 90% for persons aged >65 years (objective no. 14-29a) (125). Among persons aged >65 years, influenza vaccination levels increased from 33% in 1989 (126) to 66% in 1999 (127), surpassing the Healthy People 2000 objective of 60% (128). Vaccination coverage in this group reached the highest levels recorded (68%) during the 1999--00 influenza season. This estimate was made using the percentage of adults reporting influenza vaccination during the previous 12 months in the National Health Interview Survey (NHIS). The NHIS administered during the first and second quarters of each calendar year was used as a proxy measure of influenza vaccination coverage for the previous influenza season (127). Possible reasons for increases in influenza vaccination levels among persons aged >65 years include 1) greater acceptance of preventive medical services by practitioners; 2) increased delivery and administration of vaccine by health-care providers and sources other than physicians; 3) new information regarding influenza vaccine effectiveness, cost-effectiveness, and safety; and 4) initiation of Medicare reimbursement for influenza vaccination in 1993 (8,14,15,101,102,129,130). Since 1997, influenza vaccination levels have increased more slowly, with an average annual percentage increase of 4% from 1988--89 to 1996--97 versus 1% from 1996--97 to 1998--99. In 2000, a substantial delay in influenza vaccine availability and distribution, followed by a less severe delay in 2001 likely contributed to the lack of progress. However, the slowing of the increase in vaccination levels began before 2000 and is not fully understood. Estimated national influenza vaccine coverage in 2004 among persons aged >65 years and 50--64 years was 65% and 36%, respectively, based on 2004 NHIS data (Table 3). The estimated vaccination coverage among adults with high-risk conditions aged 18--49 years and 50--64 years was 26% and 46%, respectively, substantially lower than the Healthy People 2000 and 2010 objective of 60% (125,128). Continued annual monitoring is needed to determine the effects of vaccine supply delays and shortages, changes in influenza vaccination recommendations and target groups for vaccination, reimbursement rates for vaccine and vaccine administration, and other factors related to vaccination coverage among adults and children. New strategies to improve coverage will be needed to achieve the Healthy People 2010 objective (21,22). Reducing racial and ethnic health disparities, including disparities in vaccination coverage, is an overarching national goal (125). Although estimated influenza vaccination coverage for the 1999--00 season reached the highest levels recorded among older black, Hispanic, and white populations, vaccination levels among blacks and Hispanics continue to lag behind those among whites (127,131). Estimated vaccination coverage levels based on 2004 NHIS data among persons aged >65 years were 67% among non-Hispanic whites, 45% among non-Hispanic blacks, and 55% among Hispanics (CDC, unpublished data, 2006). Among Medicare beneficiaries, unequal access to care might not be the only factor in contributing toward disparity levels in influenza vaccination; other key factors include having patients that actively seek vaccination and providers that recommend vaccination (132,133). In 1997 and 1998, vaccination coverage estimates among nursing home residents were 64%--82% and 83%, respectively (134,135). The Healthy People 2010 goal is to achieve influenza vaccination of 90% among nursing home residents, an increase from the Healthy People 2000 goal of 80% (125,128). Reported vaccination levels are low among children at increased risk for influenza complications. One study conducted among patients in health maintenance organizations (HMOs) documented influenza vaccination percentages ranging from 9% to 10% among children with asthma (136). A 25% vaccination level was reported among children with severe to moderate asthma who attended an allergy and immunology clinic (137). However, a study conducted in a pediatric clinic demonstrated an increase in the vaccination percentage of children with asthma or reactive airways disease from 5% to 32% after implementing a reminder/recall system (138). One study documented 79% vaccination coverage among children attending a cystic fibrosis treatment center (139). According to 2004 National Immunization Survey data, during the second year of the encouragement for vaccination of children aged 6--23 months, 18% received one or more influenza vaccinations and 8.4% received 2 doses if they were previously unvaccinated (140). A rapid analysis of influenza vaccination coverage levels among members of an HMO in Northern California determined that in 2004--05, the first year of the recommendation for vaccination of children aged 6--23 months, their coverage level reached 57% (141). Data from the Behavioral Risk Factor Surveillance System (BRFSS) collected in February 2005 indicated a national estimate of 48% vaccination coverage for 1 or more doses among children aged 6--23 months and 35% coverage among children aged 2--17 years who had one or more high-risk medical conditions during the 2004--05 season (142). Increasing vaccination coverage among persons who have high-risk conditions and are aged <65 years, including children at high risk, is the highest priority for expanding influenza vaccine use. As has been observed for older adults, a physician recommendation for vaccination and the perception that getting a child vaccinated "is a smart idea" were positively associated with likelihood of vaccination of children aged 6--23 months (143). Annual vaccination is recommended for health-care workers. Nonetheless, NHIS 2004 survey data indicated a vaccination coverage level of only 42% among health-care workers (CDC, unpublished data, 2006). Vaccination of health-care workers has been associated with reduced work absenteeism (9) and fewer deaths among nursing home patients (144,145) and is a high priority for reducing the effect of influenza in health-care settings and for expanding influenza vaccine use (146,147). Limited information is available regarding use of influenza vaccine among pregnant women. Among women aged 18--44 years without diabetes responding to the 2001 BRFSS, those who were pregnant were less likely to report influenza vaccination during the previous 12 months (13.7%) than those women who were not pregnant (16.8%); these differences were statistically significant (148). Only 13% of pregnant women reported vaccination according to 2004 NHIS data, excluding pregnant women who reported diabetes, heart disease, lung disease, and other selected high-risk conditions (CDC, unpublished data, 2006) (Table 3). These data indicate low compliance with the ACIP recommendations for pregnant women. In a study of influenza vaccine acceptance by pregnant women, 71% who were offered the vaccine chose to be vaccinated (149). However, a 1999 survey of obstetricians and gynecologists determined that only 39% administered influenza vaccine to obstetric patients, although 86% agreed that pregnant women's risk for influenza-related morbidity and mortality increases during the last two trimesters (150). Data indicate that self-report of influenza vaccination among adults, compared with extraction from the medical record, is both a sensitive and specific source of information (151). Patient self-reports should be accepted as evidence of influenza vaccination in clinical practice (151). However, information on the validity of parents' reports of pediatric influenza vaccination is not yet available. Recommendations for Using Inactivated and Live, Attenuated Influenza VaccinesThe inactivated influenza vaccine and LAIV can be used to reduce the risk for influenza virus infection and its complications. TIV is Food and Drug Administration (FDA)-approved for persons aged >6 months, including those with high-risk conditions, whereas LAIV is approved only for use among healthy persons aged 5--49 years (see Inactivated Influenza Vaccine Recommendations; and Live, Attenuated Influenza Vaccine Recommendations). Target Groups for VaccinationAnnual influenza vaccination is recommended for the following groups: Persons at Increased Risk for Complications Vaccination with inactivated influenza vaccine is recommended for the following persons who are at increased risk for severe complications from influenza:
Vaccination with inactivated influenza vaccine also is recommended for the following persons because of an increased risk for influenza-associated clinic, emergency department, or hospital visits, particularly if they have a high-risk medical condition:
Persons Who Live With or Care for Persons at High Risk for Influenza-Related Complications In addition, to prevent transmission to persons identified above, vaccination with TIV or LAIV is recommended for the following persons, unless contraindicated:
In 2006, approximately 218.1 million persons in the United States will be included in one or more of these target groups, including 6.0 million children aged 6--23 months, 10.6 million healthy children aged 24--59 months, 44.0 million persons aged 2--64 years with one or more conditions associated with an increased risk for influenza-related complications, 4.0 million pregnant women, 33.0 million healthy persons aged 50--64 years, approximately 2 million nursing home residents, 37.2 million persons aged >65 years, 94.8 million healthy household contacts, and 7.0 million health-care workers aged <65 years (CDC, unpublished data, 2006). Additional Information Regarding Vaccination of Specific PopulationsHealthy Young Children Aged 6--59 Months Because children aged 6--23 months are at substantially increased risk for influenza-related hospitalizations and because children aged 24--59 months are at increased risk for influenza-related clinic and emergency department visits (152), ACIP recommends vaccination of children aged 6--59 months. The current LAIV and inactivated influenza vaccines are not approved by FDA for use among children aged <6 months, the pediatric group at greatest risk for influenza-related complications (58,153,154). Vaccination of their household contacts and out-of-home caregivers also is recommended because it might decrease the probability of influenza virus infection among these children. Studies indicate that rates of hospitalization are higher among young children than older children when influenza viruses are in circulation (57,59--61,62,155--157). The increased rates of hospitalization are comparable with rates for other groups considered at high risk for influenza-related complications. However, the interpretation of these findings has been confounded by cocirculation of respiratory syncytial virus that causes serious respiratory viral illness among children and that frequently circulates during the same time as influenza viruses (158--160). One study assessed rates of influenza-associated hospitalizations among the entire U.S. population during 1979--2001 and calculated an average rate of approximately 108 hospitalizations per 100,000 person-years in children aged <5 years (48). Two studies have attempted to separate the impact of respiratory syncytial viruses and influenza viruses on rates of hospitalization among children who do not have high-risk conditions (58,59). Both studies indicated that otherwise healthy children aged <2 years and possibly children aged 2--4 years are at increased risk for influenza-related hospitalization compared with older healthy children (Table 1). Among the Tennessee Medicaid population during 1973--1993, healthy children aged 6 months--2 years had rates of influenza-associated hospitalization comparable with or higher than rates among children aged 3--14 years with high-risk conditions (58,60). Another Tennessee study indicated a hospitalization rate per year of 3--4/1,000 healthy children aged <2 years for laboratory-confirmed influenza (36). The ability of providers to implement the recommendation to vaccinate all children aged 24--59 months during the 2006--07 season, the first year the recommendation will be in place, might vary depending upon vaccine supply (See Influenza Vaccine Supply and Timing of Annual Influenza Vaccination; and http://www.cdc.gov/nip/news/shortages/default.htm). Pregnant Women Influenza-associated excess deaths among pregnant women were documented during the pandemics of 1918--19 and 1957--58 (51,161--163). Case reports and limited studies also indicate that pregnancy can increase the risk for serious medical complications of influenza (164--169). One study of influenza vaccination of approximately 2,000 pregnant women demonstrated no adverse fetal effects associated with inactivated influenza vaccine (170); similar results were observed in a study of 252 pregnant women who received inactivated influenza vaccine within 6 months of delivery (171). No such data exist on the safety of LAIV when administered during pregnancy. Breastfeeding Mothers TIV is safe for mothers who are breastfeeding and their infants. Because excretion of LAIV in human milk is unknown and because of the possibility of shedding vaccine virus given the close proximity of a nursing mother and her infant, caution should be exercised if LAIV is administered to nursing mothers. Breastfeeding does not adversely affect the immune response and is not a contraindication for vaccination. Persons Aged 50--64 Years Vaccination is recommended for persons aged 50--64 years because this group has an increased prevalence of persons with high-risk conditions. In 2002, approximately 43.6 million persons in the United States were aged 50--64 years, of whom 13.5 million (34%) had one or more high-risk medical conditions (172). Influenza vaccine has been recommended for this entire age group to increase the low vaccination levels among persons in this age group with high-risk conditions (see Persons at Increased Risk for Complications). Age-based strategies are more successful in increasing vaccine coverage than patient-selection strategies based on medical conditions. Persons aged 50--64 years without high-risk conditions also receive benefit from vaccination in the form of decreased rates of influenza illness, decreased work absenteeism, and decreased need for medical visits and medication, including antibiotics (9--12). Furthermore, 50 years is an age when other preventive services begin and when routine assessment of vaccination and other preventive services has been recommended (173,174). Health-Care Workers and Other Persons Who Can Transmit Influenza to Those at High Risk Persons who are clinically or asymptomatically infected can transmit influenza virus to persons at high risk for complications from influenza. Decreasing transmission of influenza from caregivers and household contacts to persons at high risk might reduce influenza-related deaths among persons at high risk. In two studies, vaccination of health-care workers was associated with decreased deaths among nursing home patients (144,145), and hospital-based influenza outbreaks frequently occur where unvaccinated health-care workers are employed. Administration of LAIV has been demonstrated to reduce MAARI in contacts of vaccine recipients (175,176) and to reduce ILI-related economic and medical consequences (such as work days lost and number of health-care provider visits). In addition to health-care workers, additional groups that can transmit influenza to persons at high risk and that should be vaccinated include the following:
In addition, because children aged 0--23 months are at increased risk for influenza-related hospitalization (58--60), vaccination is recommended for their household contacts and out-of-home caregivers, particularly for contacts of children aged 0--5 months, because influenza vaccines have not been approved by FDA for use among children aged <6 months (see Healthy Young Children Aged 6--59 Months). Healthy persons aged 5--49 years in these groups who are not contacts of severely immunocompromised persons (see Live, Attenuated Influenza Vaccine Recommendations) can receive either LAIV or inactivated influenza vaccine. All other persons in this group should receive inactivated influenza vaccine. All health-care workers should be vaccinated against influenza annually (147,177,178). Facilities that employ health-care workers are strongly encouraged to provide vaccine to workers by using approaches that maximize vaccination levels. An improvement in vaccination coverage levels might help to protect health-care workers, their patients, and communities; improve prevention of influenza-associated disease and patient safety; and reduce disease burden. Influenza vaccination levels among health-care workers should be regularly measured and reported. Although vaccination levels for health-care workers are typically <40%, with moderate effort, organized campaigns can attain higher levels of vaccination among this population (146,179). In 2005, seven states had legislation requiring annual influenza vaccination of health-care workers or the signing of an informed declination (147), and 15 states had regulations regarding vaccination of health-care workers in long-term--care facilities (180). Physicians, nurses, and other workers in both hospital and outpatient-care settings, including medical emergency-response workers (e.g., paramedics and emergency medical technicians), should be vaccinated, as should employees of nursing home and chronic-care facilities who have contact with patients or residents. Persons Infected with HIV Limited information is available regarding the frequency and severity of influenza illness or the benefits of influenza vaccination among persons with HIV infection (181,182). However, a retrospective study of young and middle-aged women enrolled in Tennessee's Medicaid program determined that the risk for cardiopulmonary hospitalizations among women with HIV infection was higher during influenza seasons than during the peri-influenza periods. The risk for hospitalization was higher for HIV-infected women than for women with other well-recognized high-risk conditions, including chronic heart and lung diseases (183). Another study estimated that the risk for influenza-related death was 9.4--14.6/10,000 persons with acquired immunodeficiency syndrome (AIDS), compared with 0.09--0.10/10,000 among all persons aged 25--54 years and 6.4--7.0/10,000 among persons aged >65 years (184). Other reports indicate that influenza symptoms might be prolonged and the risk for complications from influenza increased for certain HIV-infected persons (185--187). Vaccination has been demonstrated to produce substantial antibody titers against influenza among vaccinated HIV-infected persons who have minimal AIDS-related symptoms and high CD4+ T-lymphocyte cell counts (188--191). A limited, randomized, placebo-controlled trial determined that inactivated influenza vaccine was highly effective in preventing symptomatic, laboratory-confirmed influenza virus infection among HIV-infected persons with a mean of 400 CD4+ T-lymphocyte cells/mm3; a limited number of persons with CD4+ T-lymphocyte cell counts of <200 were included in that study (192). A nonrandomized study among HIV-infected persons determined that influenza vaccination was most effective among persons with >100 CD4+ cells and among those with <30,000 viral copies of HIV type-1/mL (187). Among persons who have advanced HIV disease and low CD4+ T-lymphocyte cell counts, inactivated influenza vaccine might not induce protective antibody titers (190,191); a second dose of vaccine does not improve the immune response in these persons (191,192). One case study determined that HIV RNA (ribonucleic acid) levels increased transiently in one HIV-infected person after influenza virus infection (193). Studies have demonstrated a transient (i.e., 2--4 week) increase in replication of HIV-1 in the plasma or peripheral blood mononuclear cells of HIV-infected persons after vaccine administration (190,194). Other studies using similar laboratory techniques have not documented a substantial increase in the replication of HIV (195--198). Deterioration of CD4+ T-lymphocyte cell counts or progression of HIV disease has not been demonstrated among HIV-infected persons after influenza vaccination compared with unvaccinated persons (191,199). Limited information is available concerning the effect of antiretroviral therapy on increases in HIV RNA levels after either natural influenza virus infection or influenza vaccination (181,200). Because influenza can result in serious illness and because vaccination with inactivated influenza vaccine might result in the production of protective antibody titers, vaccination might benefit HIV-infected persons, including HIV-infected pregnant women. Therefore, influenza vaccination is recommended. Travelers The risk for exposure to influenza during travel depends on the time of year and destination. In the tropics, influenza can occur throughout the year. In the temperate regions of the Southern Hemisphere, the majority of influenza activity occurs during April--September. In temperate climate zones of the Northern and Southern Hemispheres, travelers also can be exposed to influenza during the summer, especially when traveling as part of large organized tourist groups (e.g., on cruise ships) that include persons from areas of the world where influenza viruses are circulating (201,202). Persons at high risk for complications of influenza and who were not vaccinated with influenza vaccine during the preceding fall or winter should consider receiving influenza vaccine before travel if they plan to
No information is available regarding the benefits of revaccinating persons before summer travel who were already vaccinated during the preceding fall. Persons at high risk who received the previous season's vaccine before travel should be revaccinated with the current vaccine the following fall or winter. Persons aged >50 years and persons at high risk should consult with their health-care provider before embarking on travel during the summer to discuss the symptoms and risks for influenza and other travel-related diseases. General Population In addition to the groups for which annual influenza vaccination is recommended, vaccination providers should administer influenza vaccine to any person who wishes to reduce the likelihood of becoming ill with influenza or transmitting influenza to others should they become infected (the vaccine can be administered to children aged >6 months), depending on vaccine availability (see Influenza Vaccine Supply and Timing of Annual Influenza Vaccination). A strategy of universal influenza vaccination is being assessed by ACIP. Persons who provide essential community services should be considered for vaccination to minimize disruption of essential activities during influenza outbreaks. Students or other persons in institutional settings (e.g., those who reside in dormitories) should be encouraged to receive vaccine to minimize the disruption of routine activities during epidemics (203). Inactivated Influenza Vaccine RecommendationsTIV Dosage Dosage recommendations vary according to age group (Table 4). Among previously unvaccinated children aged 6 months--<9 years, 2 doses of inactivated vaccine administered >1 month apart are recommended for eliciting satisfactory antibody responses (85--88). If possible, the second dose should be administered before the onset of influenza season. If a child aged 6 months--<9 years receiving influenza vaccine for the first time does not receive a second dose of vaccine within the same season, only 1 dose of vaccine should be administered the following season. Two doses are not required at that time. ACIP does not recommend that a child receiving influenza vaccine for the first time be administered the first dose of vaccine in the spring as a priming dose for the following season (86,88). Among adults, studies have indicated limited or no improvement in antibody response when a second dose is administered during the same season (204--206). Even when the current influenza vaccine contains one or more antigens administered in previous years, annual vaccination with the vaccine is necessary because immunity declines during the year after vaccination (207,208). Vaccine prepared for a previous influenza season should not be administered to provide protection for the current season (see Persons Who Should Not Be Vaccinated with Inactivated Influenza Vaccine). TIV Route The intramuscular route is recommended for inactivated influenza vaccine. Adults and older children should be vaccinated in the deltoid muscle. A needle length >1 inch should be considered for these age groups because needles <1 inch might be of insufficient length to penetrate muscle tissue in certain adults and older children (209). Infants and young children should be vaccinated in the anterolateral aspect of the thigh (210). ACIP recommends a needle length of 7/8--1 inch for children aged <12 months for intramuscular vaccination into the anterolateral thigh. When injecting into the deltoid muscle among children with adequate deltoid muscle mass, a needle length of 7/8--1.25 inches is recommended (210). TIV Side Effects and Adverse Reactions When educating patients regarding potential side effects, clinicians should emphasize that 1) inactivated influenza vaccine contains noninfectious killed viruses and cannot cause influenza, and 2) coincidental respiratory disease unrelated to influenza vaccination can occur after vaccination. TIV Local Reactions In placebo-controlled studies among adults, the most frequent side effect of vaccination is soreness at the vaccination site (affecting 10%--64% of patients) that lasts <2 days (12,211--213). These local reactions typically are mild and rarely interfere with the person's ability to conduct usual daily activities. One blinded, randomized, cross-over study among 1,952 adults and children with asthma demonstrated that only body aches were reported more frequently after inactivated influenza vaccine (25.1%) than placebo-injection (20.8%) (214). One study reported 20%--28% of children with asthma aged 9 months--18 years experienced local pain and swelling (81), and another study reported 23% of children aged 6 months--4 years with chronic heart or lung disease had local reactions (76). A different study reported no difference in local reactions among 53 children aged 6 months--6 years with high-risk medical conditions or among 305 healthy children aged 3--12 years in a placebo-controlled trial of inactivated influenza vaccine (77). In a study of 12 children aged 5--32 months, no substantial local or systemic reactions were noted (215). The interpretation of these findings should be made with caution given the small number of children studied. TIV Systemic Reactions Fever, malaise, myalgia, and other systemic symptoms can occur after vaccination with inactivated vaccine and most often affect persons who have had no previous exposure to the influenza virus antigens in the vaccine (e.g., young children) (216,217). These reactions begin 6--12 hours after vaccination and can persist for 1--2 days. Placebo-controlled trials demonstrate that among older persons and healthy young adults, administration of split-virus influenza vaccine is not associated with higher rates of systemic symptoms (e.g., fever, malaise, myalgia, and headache) when compared with placebo injections (12,211--213). In a randomized cross-over study among both children and adults with asthma, no increase in asthma exacerbations was reported for either age group (214). An analysis of 215,600 children aged <18 years and 8,476 children aged 6--23 months enrolled in one of five HMOs reported no increase in biologically plausible medically attended events during the 2 weeks after inactivated influenza vaccination, compared with control periods 3--4 weeks before and after vaccination (218). In a study of 791 healthy children (71), postvaccination fever was noted among 11.5% of children aged 1--5 years, among 4.6% of children aged 6--10 years, and among 5.1% of children aged 11--15 years. Among children with high-risk medical conditions, one study of 52 children aged 6 months--4 years indicated that 27% had fever and 25% had irritability and insomnia (76); another study among 33 children aged 6--18 months indicated that one child had irritability and one had a fever and seizure after vaccination (219). No placebo comparison group was used in these studies. A published review of the Vaccine Adverse Event Reporting System (VAERS) reports of TIV in children aged 6--23 months documented that the most frequently reported adverse events were fever, rash, injection-site reactions, and seizures. The majority of the small total number of reported seizures appeared to be febrile (220). Because of the limitations of passive reporting systems, determining causality for specific types of adverse events, with the exception of injection-site reactions, is usually not possible using VAERS data alone. A population-based study of TIV safety in children aged 6--23 months who were vaccinated during 1993--1999 indicated no vaccine-associated adverse events that had a plausible relationship to vaccination (221). Health-care professionals should promptly report to VAERS all clinically significant adverse events after influenza vaccination, even if the health-care professional is not certain that the vaccine caused the event. The Institute of Medicine has specifically recommended reporting of potential neurologic complications (e.g., demyelinating disorders such as Guillain-Barré syndrome [GBS]), although no evidence exists of a causal relation between influenza vaccine and neurologic disorders in children. Immediate, presumably allergic, reactions (e.g., hives, angioedema, allergic asthma, and systemic anaphylaxis) rarely occur after influenza vaccination (222). These reactions probably result from hypersensitivity to certain vaccine components; the majority of reactions probably are caused by residual egg protein. Although current influenza vaccines contain only a limited quantity of egg protein, this protein can induce immediate hypersensitivity reactions among persons who have severe egg allergy. Persons who have had hives or swelling of the lips or tongue or who have experienced acute respiratory distress or collapse after eating eggs should consult a physician for appropriate evaluation to help determine if vaccine should be administered. Persons who have documented immunoglobulin E (IgE)-mediated hypersensitivity to eggs, including those who have had occupational asthma or other allergic responses to egg protein, might also be at increased risk for allergic reactions to influenza vaccine, and consultation with a physician should be considered (223--225). Persons with a history of severe hypersensitivity (e.g., anaphylaxis) to eggs should not receive influenza vaccine. Hypersensitivity reactions to any vaccine component can occur theoretically. Although exposure to vaccines containing thimerosal can lead to induction of hypersensitivity, the majority of patients do not have reactions to thimerosal when it is administered as a component of vaccines, even when patch or intradermal tests for thimerosal indicate hypersensitivity (226,227). When reported, hypersensitivity to thimerosal usually has consisted of local, delayed hypersensitivity reactions (226). GBS and TIV The 1976 swine influenza vaccine was associated with an increased frequency of GBS (228,229). Among persons who received the swine influenza vaccine in 1976, the rate of GBS was <10 cases/1 million persons vaccinated. The risk for influenza vaccine-associated GBS was higher among persons aged >25 years than persons aged <25 years (228). Evidence for a causal relation of GBS with subsequent vaccines prepared from other influenza viruses is unclear. Obtaining strong epidemiologic evidence for a possible limited increase in risk is difficult for such a rare condition as GBS, which has an estimated annual incidence of 10--20 cases/1 million adults (230). Investigations to date have not documented a substantial increase in GBS associated with influenza vaccines (other than the swine influenza vaccine in 1976), and suggest that, if influenza vaccine does pose a risk, it is probably slightly more than one additional case/1 million persons vaccinated. During three of four influenza seasons studied during 1977--1991, the overall relative risk estimates for GBS after influenza vaccination were slightly elevated, but they were not statistically significant in any of these studies (231--233). However, in a study of the 1992--93 and 1993--94 influenza seasons, the overall relative risk for GBS was 1.7 (CI = 1.0--2.8; p = 0.04) during the 6 weeks after vaccination, representing approximately 1 additional case of GBS/1 million persons vaccinated; the combined number of GBS cases peaked 2 weeks after vaccination (234). VAERS has documented decreased reporting of postinfluenza vaccine GBS across age groups, despite overall increased reporting of other, non-GBS conditions occurring after influenza vaccination (235). Cases of GBS after influenza infection have been reported, but no other epidemiologic studies have documented such an association (236,237). Substantial evidence exists that several infectious illnesses, most notably Campylobacter jejuni and upper respiratory tract infections are associated with GBS (230,238--240). Even if GBS were a true side effect of vaccination in the years other than 1976, the estimated risk for GBS of approximately 1 additional case/1 million persons vaccinated is substantially less than the risk for severe influenza, which can be prevented by vaccination among all age groups, especially persons aged >65 years and those who have medical indications for influenza vaccination (Table 1) (see Hospitalizations and Deaths from Influenza). The potential benefits of influenza vaccination in preventing serious illness, hospitalization, and death substantially outweigh the possible risks for experiencing vaccine-associated GBS. The average case fatality ratio for GBS is 6% and increases with age (230,241). No evidence indicates that the case fatality ratio for GBS differs among vaccinated persons and those not vaccinated. The incidence of GBS among the general population is low, but persons with a history of GBS have a substantially greater likelihood of subsequently experiencing GBS than persons without such a history (231,242). Thus, the likelihood of coincidentally experiencing GBS after influenza vaccination is expected to be greater among persons with a history of GBS than among persons with no history of this syndrome. Whether influenza vaccination specifically might increase the risk for recurrence of GBS is unknown. However, avoiding vaccinating persons who are not at high risk for severe influenza complications and who are known to have experienced GBS within 6 weeks after a previous influenza vaccination is prudent. As an alternative, physicians might consider using influenza antiviral chemoprophylaxis for these persons. Although data are limited, for the majority of persons who have a history of GBS and who are at high risk for severe complications from influenza, the established benefits of influenza vaccination justify yearly vaccination. Thimerosal and Inactivated Influenza Vaccine Thimerosal, a mercury-containing compound, has been used as a preservative in vaccines since the 1930s and is used in multidose vials of inactivated influenza vaccine to reduce the likelihood of bacterial contamination (243). Many of the single-dose syringes and vials of TIV are thimerosal-free or contain only trace amounts of thimerosal (Table 4). No scientific evidence indicates that thimerosal in vaccines, including influenza vaccines, leads to serious adverse events in vaccine recipients (244). However, in 1999, the U.S. Public Health Service and other organizations recommended that efforts be made to eliminate or reduce the thimerosal content in vaccines to decrease total mercury exposure, chiefly among infants (243--245). Since mid-2001, vaccines routinely recommended for infants in the United States have been manufactured either without or with only trace amounts of thimerosal, resulting in a substantial reduction in the total mercury exposure from vaccines for children (210). Vaccines containing trace amounts of thimerosal have <1 mcg mercury/dose. The risks for severe illness from influenza virus infection are elevated among both young children and pregnant women, and persons in both groups benefit from vaccination. In contrast, no scientifically conclusive evidence exists of harm from exposure to thimerosal preservative-containing vaccine. In fact, evidence is accumulating that supports the absence of any harm resulting from exposure to such vaccines (243,246--248). Therefore, the benefits of influenza vaccination outweigh the theoretical risk, if any, from thimerosal exposure through vaccination. Nonetheless, certain persons remain concerned regarding exposure to thimerosal. As of February 2006, six states had enacted legislation banning the administration of vaccines containing mercury; the provisions defining mercury content vary. These laws might present a barrier to vaccination until sufficient numbers of doses of influenza vaccines without thimerosal as a preservative or in trace amounts are available. The U.S. vaccine supply for infants and pregnant women is in a period of transition; the availability of thimerosal-reduced or thimerosal-free vaccine intended for these groups is being expanded by manufacturers as a feasible means of reducing an infant's total exposure to mercury, because other environmental sources of exposure are more difficult or impossible to eliminate. Reductions in thimerosal in other vaccines have been achieved already and have resulted in substantially lowered cumulative exposure to thimerosal from vaccination among infants and children. For all of those reasons, persons for whom inactivated influenza vaccine is recommended may receive vaccine with or without thimerosal, depending on availability. Persons Who Should Not Be Vaccinated with Inactivated Influenza Vaccine Inactivated influenza vaccine should not be administered to persons known to have anaphylactic hypersensitivity to eggs or to other components of the influenza vaccine without first consulting a physician (see Side Effects and Adverse Reactions). Chemoprophylactic use of antiviral agents is an option for preventing influenza among such persons. However, persons who have a history of anaphylactic hypersensitivity to vaccine components but who also are at high risk for complications from influenza can benefit from vaccine after appropriate allergy evaluation and desensitization. Information regarding vaccine components is located in package inserts from each manufacturer. Persons with moderate-to-severe acute febrile illness usually should not be vaccinated until their symptoms have abated. However, minor illnesses with or without fever do not contraindicate use of influenza vaccine, particularly among children with mild upper-respiratory tract infection or allergic rhinitis. TIV and Use of Influenza Antiviral Medications As TIV contains only influenza virus subunits and no live virus, no contraindication exists to the coadministration of TIV and influenza antivirals (see sections on Chemoprophylaxis; and Control of Influenza Outbreaks in Institutions). Live, Attenuated Influenza Vaccine RecommendationsUsing LAIV LAIV is an option for vaccination of healthy, nonpregnant persons aged 5--49 years who want to avoid influenza, and those who might be in close contact with persons at high risk for severe complications, including health-care workers. During periods when inactivated vaccine is in short supply, use of LAIV is encouraged when feasible for eligible persons (including health-care workers) because use of LAIV by these persons might increase availability of inactivated vaccine for persons in groups at high risk. Possible advantages of LAIV include its potential to induce a broad mucosal and systemic immune response, its ease of administration, and the acceptability of an intranasal rather than intramuscular route of administration. LAIV Dosage and Administration LAIV is intended for intranasal administration only and should not be administered by the intramuscular, intradermal, or intravenous route. LAIV must be thawed before administration. This can be accomplished by holding an individual sprayer in the palm of the hand until thawed, with subsequent immediate administration. Alternatively, the vaccine can be thawed in a refrigerator and stored at 2ºC--8ºC for <60 hours before use. Vaccine should not be refrozen after thawing. LAIV is supplied in a prefilled single-use sprayer containing 0.5 mL of vaccine. Approximately 0.25 mL (i.e., half of the total sprayer contents) is sprayed into the first nostril while the recipient is in the upright position. An attached dose-divider clip is removed from the sprayer to administer the second half of the dose into the other nostril. If the vaccine recipient sneezes after administration, the dose should not be repeated. LAIV should be administered annually according to the following schedule:
LAIV can be administered to persons with minor acute illnesses (e.g., diarrhea or mild upper respiratory tract infection with or without fever). However, if clinical judgment indicates nasal congestion is present that might impede delivery of the vaccine to the nasopharyngeal mucosa, deferral of administration should be considered until resolution of the illness. Whether concurrent administration of LAIV with other vaccines affects the safety or efficacy of either LAIV or the simultaneously administered vaccine is unknown. In the absence of specific data indicating interference, following the ACIP general recommendations for immunization is prudent (210). Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines. Inactivated or live vaccines can be administered simultaneously with LAIV. However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered (see Persons Who Should Not Be Vaccinated with LAIV). LAIV and Use of Influenza Antiviral Medications The effect on safety and efficacy of LAIV coadministration with influenza antiviral medications has not been studied. However, because influenza antivirals reduce replication of influenza viruses, LAIV should not be administered until 48 hours after cessation of influenza antiviral therapy, and influenza antiviral medications should not be administered for 2 weeks after receipt of LAIV. LAIV Storage LAIV must be stored at -15ºC or colder. A manufacturer-supplied freezer box was formerly required for storage of LAIV in a frost-free freezer; however, the freezer box is now optional, and LAIV may now be stored in frost-free freezers without using a freezer box. LAIV can be thawed in a refrigerator and stored at 2ºC--8ºC for <60 hours before use. It should not be refrozen after thawing because of decreased vaccine potency. Shedding, Transmission, and Stability of Vaccine Viruses Available data indicate that both children and adults vaccinated with LAIV can shed vaccine viruses for >2 days after vaccination, although in lower titers than typically occur with shedding of wild-type influenza viruses. Shedding should not be equated with person-to-person transmission of vaccine viruses, although, in rare instances, shed vaccine viruses can be transmitted from vaccinees to nonvaccinated persons. One unpublished study of a child care center setting assessed transmissibility of vaccine viruses from 98 vaccinated to 99 unvaccinated children, all aged 8--36 months. Eighty percent of vaccine recipients shed one or more virus strains, with a mean of 7.6 days' duration (249). One vaccine type influenza type B isolate was recovered from a placebo recipient and was confirmed to be vaccine-type virus. The type B isolate retained the cold-adapted, temperature-sensitive, attenuated phenotype, and it possessed the same genetic sequence as a virus shed from a vaccine recipient in the same children's play group. The placebo recipient from whom the influenza type B vaccine virus was isolated did not exhibit symptoms that were different from those experienced by vaccine recipients. The estimated probability of acquiring vaccine virus after close contact with a single LAIV recipient in this child care population was 0.58%--2.4%. One study assessing shedding of vaccine viruses in 20 healthy vaccinated adults aged 18--49 years demonstrated that the majority of shedding occurred within the first 3 days after vaccination, although one participant was noted to shed virus on day 7 after vaccine receipt. No study participants shed vaccine viruses >10 days after vaccination. Duration or type of symptoms associated with receipt of LAIV did not correlate with duration of shedding vaccine viruses. Person-to-person transmission of vaccine viruses was not assessed in this study (250). Another study assessing shedding of vaccine viruses in 14 healthy adults aged 18--49 years indicated that 50% of these adults had viral antigen detected by direct immunofluorescence or rapid antigen tests within 7 days of vaccination. The majority of viral shedding was detected on day 2 or 3. Person-to-person transmission of vaccine viruses was not assessed in this study (251). In clinical trials, viruses shed by vaccine recipients have been phenotypically stable. In one study, nasal and throat swab specimens were collected from 17 study participants for 2 weeks after vaccine receipt (252). Virus isolates were analyzed by multiple genetic techniques. All isolates retained the LAIV genotype after replication in the human host, and all retained the cold-adapted and temperature-sensitive phenotypes. A study conducted in a day care setting found that limited genetic change occurred in the LAIV strains after replication in the vaccine recipients (253). LAIV Side Effects and Adverse Reactions Twenty prelicensure clinical trials assessed the safety of the approved LAIV. In these combined studies, approximately 28,000 doses of the vaccine were administered to approximately 20,000 persons. A subset of these trials were randomized, placebo-controlled studies in which an estimated 4,000 healthy children aged 5--17 years and 2,000 healthy adults aged 18--49 years were vaccinated. The incidence of adverse events possibly complicating influenza (e.g., pneumonia, bronchitis, bronchiolitis, or central nervous system events) was not statistically different among LAIV and placebo recipients aged 5--49 years. LAIV is made from attenuated viruses and does not cause influenza in vaccine recipients. Children. In a subset of healthy children aged 60--71 months from one clinical trial (111,112), certain signs and symptoms were reported more often among LAIV recipients after the first dose (n = 214) than placebo recipients (n = 95) (e.g., runny nose, 48.1% versus 44.2%; headache, 17.8% versus 11.6%; vomiting, 4.7% versus 3.2%; and myalgias, 6.1% versus 4.2%), but these differences were not statistically significant. In other trials, signs and symptoms reported after LAIV administration have included runny nose or nasal congestion (20%--75%), headache (2%--46%), fever (0--26%), vomiting (3%--13%), abdominal pain (2%), and myalgias (0--21%) (105,108,110,254--256). These symptoms were associated more often with the first dose and were self-limited. Data from a study of children aged 1--17 years indicated an increase in asthma or reactive airways disease in the subset aged 1--<5 years (257,258). Because of these data, LAIV is not approved for use among children aged <5 years. Another study was conducted among more than 11,000 children aged 18 months--18 years in which 18,780 doses of vaccine were administered over a 4-year period. This study did not observe an increase in asthma visits 0--15 days after vaccination for children who were aged 18 months--4 years compared with the prevaccination period; however, a significant increase in asthma events was observed 15--42 days after vaccination but only in vaccine year 1 (259). Adults. Among adults, runny nose or nasal congestion (28%--78%), headache (16%--44%), and sore throat (15%--27%) have been reported more often among vaccine recipients than placebo recipients (114,260,261). In one clinical trial (114) among a subset of healthy adults aged 18--49 years, signs and symptoms reported more frequently among LAIV recipients (n = 2,548) than placebo recipients (n = 1,290) within 7 days after each dose included cough (13.9% versus 10.8%), runny nose (44.5% versus 27.1%), sore throat (27.8% versus 17.1%), chills (8.6% versus 6.0%), and tiredness/weakness (25.7% versus 21.6%). Safety Among Groups at High Risk from Influenza-Related Morbidity. Until additional data are acquired and analyzed, persons at high risk for experiencing complications from influenza virus infection (e.g., immunocompromised patients; patients with asthma, cystic fibrosis, or chronic obstructive pulmonary disease; or persons aged >65 years) should not be vaccinated with LAIV. Protection from influenza among these groups should be accomplished using inactivated influenza vaccine. Serious Adverse Events. Serious adverse events requiring medical attention among healthy children aged 5--17 years or healthy adults aged 18--49 years occurred at a rate of <1%. Surveillance will continue for adverse events that might not have been detected in previous studies. Reviews of reports to VAERS after vaccination of approximately 2,500,000 persons during the 2003--04 and 2004--05 influenza seasons did not reveal any substantial new safety concerns (262,263). Health-care professionals should promptly report all clinically significant adverse events after LAIV administration to VAERS, as recommended for inactivated influenza vaccine. Persons Who Should Not Be Vaccinated with LAIV The following populations should not be vaccinated with LAIV:
Vaccination of Close Contacts of Persons at High Risk for Complications from Influenza Close contacts of persons at high risk for complications from influenza should receive influenza vaccine to reduce transmission of wild-type influenza viruses to persons at high risk. Use of inactivated influenza vaccine is preferred for vaccinating household members, health-care workers, and others who have close contact with severely immunocompromised persons (e.g., patients with hematopoietic stem cell transplants) during those periods in which the immunocompromised person requires care in a protective environment. The rationale for not using LAIV among health-care workers caring for such patients is the theoretical risk that a live, attenuated vaccine virus could be transmitted to the severely immunocompromised person. If a health-care worker receives LAIV, that worker should refrain from contact with severely immunocompromised patients for 7 days after vaccine receipt. Hospital visitors who have received LAIV should refrain from contact with severely immunocompromised persons for 7 days after vaccination; however, such persons need not be excluded from visitation of patients who are not severely immunocompromised. ACIP has not indicated a preference for inactivated influenza vaccine use by health-care workers or other persons who have close contact with persons with lesser degrees of immunodeficiency (e.g., persons with diabetes, persons with asthma taking corticosteroids, or persons infected with HIV) or for inactivated influenza vaccine use by health-care workers or other healthy persons aged 5--49 years in close contact with all other groups at high risk. Personnel Who May Administer LAIV Low-level introduction of vaccine viruses into the environment is likely unavoidable when administering LAIV. The risk for acquiring vaccine viruses from the environment is unknown but likely to be limited. Severely immunocompromised persons should not administer LAIV. However, other persons at high risk for influenza complications may administer LAIV. These include persons with underlying medical conditions placing them at high risk or who are likely to be at risk, including pregnant women, persons with asthma, and persons aged >50 years. Recommended Vaccines for Different Age GroupsWhen vaccinating children aged 6 months--3 years, health-care providers should use inactivated influenza vaccine that has been approved by FDA for this age group. Inactivated influenza vaccine from sanofi pasteur (Fluzone) is approved for use among persons aged >6 months. Inactivated influenza vaccine from Novartis, formerly Chiron (Fluvirin), is labeled in the United States for use among persons aged >4 years because data to demonstrate efficacy among younger persons have not been provided to FDA, whereas inactivated influenza vaccine from GlaxoSmithKline (FLUARIX) is labeled for use in persons aged >18 years. LAIV from MedImmune (FluMist) is approved for use by healthy persons aged 5--49 years (Table 4). Influenza Vaccine Supply and Timing of Annual Influenza VaccinationThe annual supply of influenza vaccine and the timing of its distribution cannot be guaranteed in any year. Currently, influenza vaccine manufacturers are projecting that approximately 100 million doses of influenza vaccine will be available in the United States for the 2006--07 influenza season, an amount that is approximately 16% more doses than were available for the 2005--06 season. An additional 15 million--20 million doses might be available if a new vaccine is licensed in 2006. (Information about the status of licensure of new vaccines is available at http://aapredbook.aappublications.org/news/vaccstatus.pdf.) However, influenza vaccine distribution delays or vaccine shortages remain possible in part because of the inherent critical time constraints in manufacturing the vaccine given the annual updating of the influenza vaccine strains. To ensure optimal use of available doses of influenza vaccine, health-care providers, those planning organized campaigns, and state and local public health agencies should 1) develop plans for expanding outreach and infrastructure to vaccinate more persons than last year and 2) develop contingency plans for the timing and prioritization of administering influenza vaccine, if the supply of vaccine is delayed and/or reduced. CDC and other public health agencies will assess the vaccine supply on a continuing basis throughout the manufacturing period and will inform both providers and the general public if a substantial delay or an inadequate supply occurs. Because LAIV is approved for use in healthy persons aged 5--49 years, no recommendations exist for limiting the timing and prioritization of administering LAIV. Administration of LAIV is encouraged as soon as it is available and throughout the season. If the supply of inactivated influenza vaccine is adequate and a sufficient number of doses will be available beginning in September, vaccination efforts should be structured to ensure the vaccination of as many persons as possible over the course of several months. Even if vaccine distribution begins in September, distribution probably will not be completed until December or January; therefore, the following recommendations reflect this phased distribution during the months of October, November, and December, and possibly later. The prioritized (tiered) use of influenza vaccine during inactivated influenza vaccine shortages applies only to the use of inactivated vaccine and not to LAIV. When feasible, during shortages of inactivated influenza vaccine, LAIV should be used preferentially for all healthy persons aged 5--49 years (including health-care workers) to increase the availability of inactivated vaccine for groups at high risk. The following section provides guidance regarding the timing of vaccination under two scenarios: 1) if the supply of inactivated influenza vaccine is adequate, and 2) if a reduced or delayed supply of inactivated vaccine occurs. Materials to assist providers are available at http://www.cdc.gov/flu/professionals/vaccination/index.htm (see also Travelers section). Vaccination Before October To avoid missed opportunities for vaccination of persons at increased risk for serious complications and their household contacts (including out-of-home caregivers and household contacts of children aged 0--59 months), such persons should be offered vaccine beginning in September during routine health-care visits or during hospitalizations, if vaccine is available. However, in facilities housing older persons (e.g., nursing homes), vaccination before October typically should be avoided because antibody levels in such persons can begin to decline more rapidly after vaccination (264). If vaccine supplies are sufficient, vaccination of other persons also may begin before October. In addition, because children aged 6 months--<9 years who have not been previously vaccinated need 2 doses of vaccine, they should receive their first dose in September, if vaccine is available, so that both doses can be administered before the onset of influenza activity. For previously vaccinated children, only 1 dose is needed. Vaccination in October and November The optimal time for vaccination efforts is usually during October--November. In October, vaccination in provider-based settings should start or continue for all patients---both high risk and healthy---and extend throughout November. Vaccination of children aged 6 months--<9 years who are receiving vaccine for the first time should also begin in October, if not done earlier, because those children need a booster dose 4--10 weeks after the initial dose, depending upon whether they are receiving inactivated influenza vaccine or LAIV. If supplies of inactivated influenza vaccine are not adequate, ACIP recommends that vaccine providers focus their vaccination efforts in October, primarily on persons aged >50 years, persons aged <50 years at increased risk for influenza-related complications (including children aged 6--59 months), household contacts of persons at high risk (including out-of-home caregivers and household contacts of children aged 0--59 months), and health-care workers (178). Efforts to vaccinate other persons who wish to decrease their risk for influenza virus infection should not begin until November; however, if such persons request vaccination in October, vaccination should not be deferred, unless vaccine supplies dictate otherwise. Vaccination in December and Later When inactivated vaccine is delayed, a substantial proportion of doses often do not become available until December or later. Nevertheless, even when supply is not delayed or reduced, as demonstrated by the relatively low vaccination coverage levels among persons in the defined priority groups, many persons who should receive influenza vaccine remain unvaccinated (Table 3). Providers should routinely offer influenza vaccine throughout the influenza season even after influenza activity has been documented in the community. In the United States, seasonal influenza activity can begin to increase as early as October or November, but influenza activity has not reached peak levels until late December--early March in the majority of recent seasons (Table 5). Although the timing of influenza activity can vary by region, vaccine administered after November is likely to be beneficial in the majority of influenza seasons. Adults have peak antibody protection against influenza virus infection 2 weeks after vaccination (265,266). Timing of Organized Vaccination Campaigns Persons and institutions planning substantial organized vaccination campaigns (e.g., health departments, occupational health clinics, and community vaccinators) should consider scheduling these events after at least mid-October because the availability of vaccine in any location cannot be ensured consistently in early fall. Scheduling campaigns after mid-October will minimize the need for cancellations because vaccine is unavailable. These vaccination clinics should be scheduled through November, with attention to settings that serve children aged 6--59 months, pregnant women, other persons aged <50 years at increased risk for influenza-related complications, persons aged >50 years, health-care workers, and household contacts and out-of-home caregivers of persons at high risk (including children aged 0--59 months) to the extent feasible. Planners are encouraged to schedule at least one vaccination clinic in December. During a vaccine shortage or delay, substantial proportions of inactivated influenza vaccine doses may not be released until November and December or later. Beginning in November, vaccination campaigns can be broadened to include healthy persons who wish to reduce their risk for influenza virus infection. ACIP recommends organizers schedule these vaccination clinics throughout November and December. When the vaccine is significantly delayed, agencies should consider offering vaccination clinics into January as long as vaccine supplies are available. Campaigns using LAIV are optimally conducted in October and November but can also extend into January. Strategies for Implementing Vaccination Recommendations in Health-Care SettingsSuccessful vaccination programs combine publicity and education for health-care workers and other potential vaccine recipients, a plan for identifying persons at high risk, use of reminder/recall systems, assessment of practice-level vaccination rates with feedback to staff, and efforts to remove administrative and financial barriers that prevent persons from receiving the vaccine, including use of standing orders programs (19,267). Since October 2005, the Centers for Medicare and Medicaid Services (CMS) has required nursing homes participating in the Medicare and Medicaid programs to offer all residents influenza and pneumococcal vaccines and to document the results. According to the requirements, each resident is to be vaccinated unless it is medically contraindicated or the resident or his/her legal representative refuses vaccination. This information is to be reported as part of the CMS Minimum Data Set, which tracks nursing home health parameters (268). The use of standing orders programs by long-term--care facilities (e.g., nursing homes and skilled nursing facilities), hospitals, and home health agencies might help to ensure the administration of recommended vaccinations for adults (269). Standing orders programs for both influenza and pneumococcal vaccination should be conducted under the supervision of a licensed practitioner according to a physician-approved facility or agency policy by health-care workers trained to screen patients for contraindications to vaccination, administer vaccine, and monitor for adverse events. CMS has removed the physician signature requirement for the administration of influenza and pneumococcal vaccines to Medicare and Medicaid patients in hospitals, long-term--care facilities, and home health agencies (269). To the extent allowed by local and state law, these facilities and agencies may implement standing orders for influenza and pneumococcal vaccination of Medicare- and Medicaid-eligible patients. Other settings (e.g., outpatient facilities, managed care organizations, assisted living facilities, correctional facilities, pharmacies, and adult workplaces) are encouraged to introduce standing orders programs as well (20). In addition, physician reminders (e.g., flagging charts) and patient reminders are recognized strategies for increasing rates of influenza vaccination. Persons for whom influenza vaccine is recommended can be identified and vaccinated in the settings described in the following sections. Outpatient Facilities Providing Ongoing Care Staff in facilities providing ongoing medical care (e.g., physicians' offices, public health clinics, employee health clinics, hemodialysis centers, hospital specialty-care clinics, and outpatient rehabilitation programs) should identify and label the medical records of patients who should receive vaccination. Vaccine should be offered during visits beginning in September (if vaccine is available) and throughout the influenza season. The offer of vaccination and its receipt or refusal should be documented in the medical record. Patients for whom vaccination is recommended and who do not have regularly scheduled visits during the fall should be reminded by mail, telephone, or other means of the need for vaccination. Outpatient Facilities Providing Episodic or Acute Care Beginning each September, acute health-care facilities (e.g., emergency departments and walk-in clinics) should offer vaccinations to persons for whom vaccination is recommended or provide written information regarding why, where, and how to obtain the vaccine. This written information should be available in languages appropriate for the populations served by the facility. Nursing Homes and Other Residential Long-Term--Care Facilities During October and November each year, vaccination should be routinely provided to all residents of chronic-care facilities with the concurrence of attending physicians. Consent for vaccination should be obtained from the resident or a family member at the time of admission to the facility or anytime afterwards. Ideally, all residents should be vaccinated at one time, before influenza season. Residents admitted through March after completion of the vaccination program at the facility should be vaccinated at the time of admission. Acute-Care Hospitals Persons of all ages (including children) with high-risk conditions and persons aged >50 years who are hospitalized at any time during September--March should be offered and strongly encouraged to receive influenza vaccine before they are discharged if they have not already received the vaccine during that season. In one study, 39%--46% of adult patients hospitalized during the winter with influenza-related diagnoses had been hospitalized during the preceding fall (270). Thus, the hospital serves as a setting in which persons at increased risk for subsequent hospitalization can be identified and vaccinated. However, vaccination of persons at high risk during or after their hospitalizations is often not done. In a study of hospitalized Medicare patients, only 31.6% were vaccinated before admission, 1.9% during admission, and 10.6% after admission (271). Using standing orders in hospitals increases vaccination rates among hospitalized persons (272). Visiting Nurses and Others Providing Home Care to Persons at High Risk Beginning in September, nursing-care plans should identify patients for whom vaccination is recommended, and vaccine should be administered in the home, if necessary. Caregivers and other persons in the household (including children) should be referred for vaccination. Other Facilities Providing Services to Persons Aged >50 Years Beginning in October, such facilities as assisted living housing, retirement communities, and recreation centers should offer unvaccinated residents and attendees vaccination on-site before the start of the influenza season. Staff education should emphasize the need for influenza vaccine. Health-Care Workers Beginning in October each year, health-care facilities should offer influenza vaccinations to all workers, including night and weekend staff. Particular emphasis should be placed on providing vaccinations to persons who care for members of groups at high risk. Efforts should be made to educate health-care workers regarding the benefits of vaccination and the potential health consequences of influenza illness for their patients, themselves, and their family members. All health-care workers should be provided convenient access to influenza vaccine at the work site, free of charge, as part of employee health programs (146,177,179). Future Directions for Research and Recommendations Related to Influenza VaccineThe relatively low effectiveness of influenza vaccine administered to older adults highlights the need for more immunogenic influenza vaccines for the elderly (273) and the need for additional research to understand potential biases in estimating the benefits of vaccination among older adults in reducing hospitalizations and deaths (274--276). Additional studies of the relative cost-effectiveness and cost utility of influenza vaccination among children and adults, especially those aged <65 years, are needed and should be designed to account for year-to-year variations in influenza attack rates, illness severity, hospitalization costs and rates, and vaccine effectiveness (277). Additional data also are needed to quantify the benefits of influenza vaccination of health-care workers in protecting their patients (278). Furthermore, larger consortia of networks are needed that are able to assess rare events that occur after vaccination, including GBS. ACIP continues to review new vaccination strategies to protect against influenza, including the possibility of expanding routine influenza vaccination recommendations toward universal vaccination or other approaches that will help greatly reduce or prevent the transmission of influenza (279--282). In addition, as noted by the National Vaccine Advisory Committee, strengthening the U.S. influenza vaccination system will require improving vaccine financing, increasing demand, and implementing systems to help better understand the burden of influenza in the United States (283). Strategies to evaluate the effect of vaccination recommendations remain critical. Recommendations for Using Antiviral Agents for InfluenzaAlthough annual vaccination is the primary strategy for preventing complications of influenza virus infections, antiviral medications with activity against influenza viruses can be effective for the chemoprophylaxis and treatment of influenza. Four licensed influenza antiviral agents are available in the United States: amantadine, rimantadine, zanamivir, and oseltamivir. Influenza A virus resistance to amantadine and rimantadine can emerge rapidly during treatment. On the basis of antiviral testing results conducted at CDC and in Canada indicating high levels of resistance (23,24,284), ACIP recommends that neither amantadine nor rimantadine be used for the treatment or chemoprophylaxis of influenza A in the United States until susceptibility to these antiviral medications has been re-established among circulating influenza A viruses. Oseltamivir or zanamivir can be prescribed if antiviral treatment of influenza is indicated. Oseltamivir is approved for treatment of persons aged >1 year, and zanamivir is approved for treatment of persons aged >7 years. Oseltamivir and zanamivir can be used for chemoprophylaxis of influenza; oseltamivir is licensed for use in persons aged >1 year, and zanamivir is licensed for use in persons aged >5 years. Antiviral Agents for InfluenzaZanamivir and oseltamivir are chemically related antiviral drugs known as neuraminidase inhibitors that have activity against both influenza A and B viruses. Both zanamivir and oseltamivir were approved in 1999 for treatment of uncomplicated influenza virus infections. In 2000, oseltamivir was approved for chemoprophylaxis of influenza among persons aged >13 years and was approved for chemoprophylaxis of children aged >1 year in 2005. In 2006, zanamivir was approved for chemoprophylaxis of children aged >5 years. The two drugs differ in pharmacokinetics, side effects, routes of administration, approved age groups, dosages, and costs. An overview of the indications, use, administration, and known primary side effects of these medications is presented in the following sections. Package inserts should be consulted for additional information. Detailed information regarding amantadine and rimantadine is available in the previous publication of the ACIP influenza recommendations (285). Role of Laboratory DiagnosisAppropriate treatment of patients with respiratory illness depends on accurate and timely diagnosis. Influenza surveillance information and diagnostic testing can aid clinical judgment and help guide treatment decisions. For example, early diagnosis of influenza can reduce the inappropriate use of antibiotics and provide the option of using antiviral therapy. However, because certain bacterial infections can produce symptoms similar to influenza, bacterial infections should be considered and appropriately treated, if suspected. In addition, bacterial infections can occur as a complication of influenza. The accuracy of clinical diagnosis of influenza on the basis of symptoms alone is limited because symptoms from illness caused by other pathogens can overlap considerably with influenza (33,42,43). Because testing all patients who might have influenza is not feasible, influenza surveillance by state and local health departments and CDC can provide information regarding the presence of influenza viruses in the community. Surveillance also can identify the predominant circulating types, influenza A subtypes, and strains of influenza viruses. Diagnostic tests available for influenza include viral culture, serology, rapid antigen testing, polymerase chain reaction (PCR), and immunofluorescence assays (28). The sensitivity and specificity of any test for influenza can vary by the laboratory that performs the test, the type of test used, the type of specimen tested, and the timing of specimen collection. Among respiratory specimens for viral isolation or rapid detection, nasopharyngeal specimens are typically more effective than throat swab specimens (286). As with any diagnostic test, results should be evaluated in the context of other clinical and epidemiologic information available to health-care providers. Commercial rapid diagnostic tests are available that can detect influenza viruses in 30 minutes (28,287). Some tests are approved for use in any outpatient setting, whereas others must be used in a moderately complex clinical laboratory. These rapid tests differ in the types of influenza viruses they can detect and whether they can distinguish between influenza types. Different tests can detect 1) only influenza A viruses; 2) both influenza A and B viruses, but not distinguish between the two types; or 3) both influenza A and B and distinguish between the two. None of the rapid tests provide any information regarding influenza A subtypes. The types of specimens acceptable for use (i.e., throat, nasopharyngeal, or nasal; and aspirates, swabs, or washes) also vary by test. The specificity and, in particular, the sensitivity of rapid tests are lower than for viral culture and vary by test (288,289). Because of the lower sensitivity of the rapid tests, physicians should consider confirming negative tests with viral culture or other means because of the possibility of false-negative rapid test results, especially during periods of peak community influenza activity. In contrast, false-positive rapid test results are less likely but can occur during periods of low influenza activity. Therefore, when interpreting results of a rapid influenza test, physicians should consider the positive and negative predictive values of the test in the context of the level of influenza activity in their community. Package inserts and the laboratory performing the test should be consulted for more details regarding use of rapid diagnostic tests. Additional information concerning diagnostic testing is available at http://www.cdc.gov/flu/professionals/labdiagnosis.htm. Despite the availability of rapid diagnostic tests, collecting clinical specimens for viral culture is critical because only culture isolates can provide specific information regarding circulating strains and subtypes of influenza viruses. This information is needed to compare current circulating influenza strains with vaccine strains, to guide decisions regarding influenza treatment and chemoprophylaxis, and to formulate vaccine for the coming year. Virus isolates also are needed to monitor the emergence of antiviral resistance and the emergence of novel influenza A subtypes that might pose a pandemic threat. Antiviral Drug-Resistant Strains of Influenza VirusCDC recently reported that 193 (92%) of 209 influenza A (H3N2) viruses isolated from patients in 26 states demonstrated a change at amino acid 31 in the M2 gene that confers resistance to adamantanes (23,24). In addition, two of eight influenza A (H1N1) viruses tested were resistant (24). Canadian health authorities also have reported the same mutation in a comparable proportion of isolates recently tested (284). Until these findings, previous screenings of epidemic strains of influenza A viruses found few amantadine- and rimantadine-resistant viruses (290--292). Viral resistance to adamantanes can emerge rapidly during treatment because a single point mutation at amino acid positions 26, 27, 30, 31, or 34 of the M2 protein can confer cross resistance to both amantadine and rimantadine (293,294). Drug-resistant viruses can emerge in approximately one third of patients when either amantadine or rimantadine is used for therapy (293,295,296). During the course of amantadine or rimantadine therapy, resistant influenza strains can replace susceptible strains within 2--3 days of starting therapy (290,297). Resistant viruses have been isolated from persons who live at home or in an institution in which other residents are taking or have taken amantadine or rimantadine as therapy (298,299); however, the frequency with which resistant viruses are transmitted and their effect on efforts to control influenza are unknown. Persons who have influenza A virus infection and who are treated with either amantadine or rimantadine can shed susceptible viruses early in the course of treatment and later shed drug-resistant viruses, including after 5--7 days of therapy (295). Resistance to zanamivir and oseltamivir can be induced in influenza A and B viruses in vitro (300--307), but induction of resistance usually requires multiple passages in cell culture. By contrast, resistance to amantadine and rimantadine in vitro can be induced with fewer passages in cell culture (308,309). Development of viral resistance to zanamivir and oseltamivir during treatment has been identified but does not appear to be frequent (310--314). In one pediatric study, 5.5% of patients treated with oseltamivir had posttreatment isolates that were resistant to neuraminidase inhibitors. One small study of Japanese children treated with oseltamivir reported a high frequency of resistant viruses (315). However, no transmission of neuraminidase inhibitor-resistant viruses in humans has been documented to date. No isolates with reduced susceptibility to zanamivir have been reported from clinical trials, although the number of posttreatment isolates tested is limited (316), and the risk for emergence of zanamivir-resistant isolates cannot be quantified (317). Only one clinical isolate with reduced susceptibility to zanamivir, obtained from an immunocompromised child on prolonged therapy, has been reported (312). Available diagnostic tests are not optimal for detecting clinical resistance to the neuraminidase inhibitor antiviral drugs, and additional tests are being developed (316,318). Postmarketing surveillance for neuraminidase inhibitor-resistant influenza viruses is being conducted (319). Indications for Use of Antivirals When Susceptibility ExistsTreatment When administered within 2 days of illness onset to otherwise healthy adults, zanamivir and oseltamivir can reduce the duration of uncomplicated influenza A and B illness by approximately 1 day compared with placebo (91,320--334). More clinical data are available concerning the efficacy of zanamivir and oseltamivir for treatment of influenza A virus infection than for treatment of influenza B virus infection (324,335--344). However, in vitro data and studies of treatment among mice and ferrets (345--352), in addition to clinical studies, have documented that zanamivir and oseltamivir have activity against influenza B viruses (310,317,325,329,353,354). Data are limited regarding the effectiveness of the antiviral agents in preventing serious influenza-related complications (e.g., bacterial or viral pneumonia or exacerbation of chronic diseases). Evidence for the effectiveness of these antiviral drugs is principally based on studies of patients with uncomplicated influenza (355). Data are limited concerning the effectiveness of zanamivir and oseltamivir for treatment of influenza among persons at high risk for serious complications of influenza (31,321,322,324,325,330--338). Among influenza virus infected participants in 10 clinical trials, the risk for pneumonia among those participants receiving oseltamivir was approximately 50% lower than among those persons receiving a placebo (339). A similar significant reduction was also found for hospital admissions; a 50% reduction was observed in the small subset of high-risk participants, although this reduction was not statistically significant. Fewer studies of the efficacy of influenza antivirals have been conducted among pediatric populations (295,322,328,329). One study of oseltamivir treatment documented a decreased incidence of otitis media among children (323). Inadequate data exist regarding the safety and efficacy of any of the influenza antiviral drugs for use among children aged <1 year (289). Initiation of antiviral treatment within 2 days of illness onset is recommended. The recommended duration of treatment with either zanamivir or oseltamivir is 5 days. Chemoprophylaxis Chemoprophylactic drugs are not a substitute for vaccination, although they are critical adjuncts in preventing and controlling influenza. In community studies of healthy adults, both oseltamivir and zanamivir are similarly effective in preventing febrile, laboratory-confirmed influenza illness (efficacy: zanamivir, 84%; oseltamivir, 82%) (324,340,356). Both antiviral agents also have been reported to prevent influenza illness among persons administered chemoprophylaxis after a household member had influenza diagnosed (341,353,356). Experience with chemoprophylactic use of these agents in institutional settings or among patients with chronic medical conditions is limited in comparison with the adamantanes (310,337,338,342--344). One 6-week study of oseltamivir chemoprophylaxis among nursing home residents reported a 92% reduction in influenza illness (310,357). Use of zanamivir has not been reported to impair the immunologic response to influenza vaccine (317,358). Data are not available regarding the efficacy of any of the four antiviral agents in preventing influenza among severely immunocompromised persons. When determining the timing and duration for administering influenza antiviral medications for chemoprophylaxis, factors related to cost, compliance, and potential side effects should be considered. To be maximally effective as chemoprophylaxis, the drug must be taken each day for the duration of influenza activity in the community. Persons at High Risk Who Are Vaccinated After Influenza Activity Has Begun. Persons at high risk for complications of influenza still can be vaccinated after an outbreak of influenza has begun in a community. However, development of antibodies in adults after vaccination takes approximately 2 weeks (265,266). When influenza vaccine is administered while influenza viruses are circulating, chemoprophylaxis should be considered for persons at high risk during the time from vaccination until immunity has developed. Children aged <9 years who receive influenza vaccine for the first time can require 6 weeks of chemoprophylaxis (i.e., chemoprophylaxis for 4 weeks after the first dose of vaccine and an additional 2 weeks of chemoprophylaxis after the second dose). Persons Who Provide Care to Those at High Risk. To reduce the spread of virus to persons at high risk during community or institutional outbreaks, chemoprophylaxis during peak influenza activity can be considered for unvaccinated persons who have frequent contact with persons at high risk. Persons with frequent contact include employees of hospitals, clinics, and chronic-care facilities; household members; visiting nurses; and volunteer workers. If an outbreak is caused by a strain of influenza that might not be covered by the vaccine, chemoprophylaxis should be considered for all such persons, regardless of their vaccination status. Persons Who Have Immune Deficiencies. Chemoprophylaxis can be considered for persons at high risk who are expected to have an inadequate antibody response to influenza vaccine. This category includes persons infected with HIV, chiefly those with advanced HIV disease. No published data are available concerning possible efficacy of chemoprophylaxis among persons with HIV infection or interactions with other drugs used to manage HIV infection. Such patients should be monitored closely if chemoprophylaxis is administered. Other Persons. Chemoprophylaxis throughout the influenza season or during peak influenza activity might be appropriate for persons at high risk who should not be vaccinated. Chemoprophylaxis also can be offered to persons who wish to avoid influenza illness. Health-care providers and patients should make this decision on an individual basis. Control of Influenza Outbreaks in Institutions Using antiviral drugs for treatment and chemoprophylaxis of influenza is a key component of influenza outbreak control in institutions. In addition to antiviral medications, other outbreak-control measures include instituting droplet precautions and establishing cohorts of patients with confirmed or suspected influenza, reoffering influenza vaccinations to unvaccinated staff and patients, restricting staff movement between wards or buildings, and restricting contact between ill staff or visitors and patients (359--361) (see Additional Information Regarding Influenza Virus Infection Control Among Specific Populations). The majority of published reports concerning use of antiviral agents to control influenza outbreaks in institutions are based on studies of influenza A outbreaks among nursing home populations that received amantadine or rimantadine (335,362--366). Less information is available concerning use of neuraminidase inhibitors in influenza A or B institutional outbreaks (337,338,344,357,367). When confirmed or suspected outbreaks of influenza occur in institutions that house persons at high risk, chemoprophylaxis should be started as early as possible to reduce the spread of the virus. In these situations, having preapproved orders from physicians or plans to obtain orders for antiviral medications on short notice can substantially expedite administration of antiviral medications. When outbreaks occur in institutions, chemoprophylaxis should be administered to all residents, regardless of whether they received influenza vaccinations during the previous fall, and should continue for a minimum of 2 weeks. If surveillance indicates that new cases continue to occur, chemoprophylaxis should be continued until approximately 1 week after the end of the outbreak. The dosage for each resident should be determined individually. Chemoprophylaxis also can be offered to unvaccinated staff members who provide care to persons at high risk. Chemoprophylaxis should be considered for all employees, regardless of their vaccination status, if the outbreak is suspected to be caused by a strain of influenza virus that is not well-matched to the vaccine. In addition to nursing homes, chemoprophylaxis also can be considered for controlling influenza outbreaks in other closed or semiclosed settings (e.g., dormitories or other settings in which persons live in close proximity). To limit the potential transmission of drug-resistant virus during outbreaks in institutions, whether in chronic or acute-care settings or other closed settings, measures should be taken to reduce contact as much as possible between persons taking antiviral drugs for treatment and other persons, including those taking chemoprophylaxis (see Antiviral Drug-Resistant Strains of Influenza Virus). DosageDosage recommendations vary by age group and medical conditions (Table 6). Children Zanamivir. Zanamivir is approved for treatment of influenza among children aged >7 years. The recommended dosage of zanamivir for treatment of influenza is two inhalations (one 5-mg blister per inhalation for a total dose of 10 mg) twice daily (approximately 12 hours apart); the chemoprophylaxis dosage of zanamivir for children aged >5 years is 10 mg (two inhalations) once a day (317). Oseltamivir. Oseltamivir is approved for treatment and chemoprophylaxis among persons aged >1 year. Recommended treatment and chemoprophylaxis dosages of oseltamivir for children vary by the weight of the child. The treatment dosage recommendation of oseltamivir for children who weigh <15 kg is 30 mg twice a day; for children weighing >15--23 kg, 45 mg twice a day; for those weighing >23--40 kg, 60 mg twice a day; and for children weighing >40 kg, 75 mg twice a day (310). The chemoprophylaxis recommended dosage of oseltamivir for children weighing <15 kg is 30 mg once a day; for those weighing >15--23 kg, 45 mg once a day; for those weighing >23--40 kg, 60 mg once a day; and for those weighing >40 kg, 75 mg once a day. Persons Aged >65 Years Zanamivir and Oseltamivir. No reduction in dosage is recommended on the basis of age alone. Persons with Impaired Renal Function Zanamivir. Limited data are available regarding the safety and efficacy of zanamivir for patients with impaired renal function. Among patients with renal failure who were administered a single intravenous dose of zanamivir, decreases in renal clearance, increases in half-life, and increased systemic exposure to zanamivir were observed (317,368). However, a limited number of healthy volunteers who received high doses of zanamivir intravenously tolerated systemic levels of zanamivir that were substantially higher than those resulting from administration of zanamivir by oral inhalation at the recommended dose (369,370). On the basis of these considerations, the manufacturer recommends no dose adjustment for inhaled zanamivir for a 5-day course of treatment for patients with either mild-to-moderate or severe impairment in renal function (317). Oseltamivir. Serum concentrations of oseltamivir carboxylate, the active metabolite of oseltamivir, increase with declining renal function (310,371). For patients with creatinine clearance of 10--30 mL/min (310), a reduction of the treatment dosage of oseltamivir to 75 mg once daily and in the chemoprophylaxis dosage to 75 mg every other day is recommended. No treatment or chemoprophylaxis dosing recommendations are available for patients undergoing routine renal dialysis treatment. Persons with Liver Disease Zanamivir and Oseltamivir. Neither of these medications has been studied among persons with hepatic dysfunction. Persons with Seizure Disorders Zanamivir and Oseltamivir. Seizure events have been reported during postmarketing use of zanamivir and oseltamivir, although no epidemiologic studies have reported any increased risk for seizures with either zanamivir or oseltamivir use. RouteOseltamivir is administered orally in capsule or oral suspension form. Zanamivir is available as a dry powder that is self-administered via oral inhalation by using a plastic device included in the package with the medication. Patients will benefit from instruction and demonstration of correct use of this device. PharmacokineticsZanamivir In studies of healthy volunteers, approximately 7%--21% of the orally inhaled zanamivir dose reached the lungs, and 70%--87% was deposited in the oropharynx (317,372). Approximately 4%--17% of the total amount of orally inhaled zanamivir is systemically absorbed. Systemically absorbed zanamivir has a half-life of 2.5--5.1 hours and is excreted unchanged in the urine. Unabsorbed drug is excreted in the feces (317,370). Oseltamivir Approximately 80% of orally administered oseltamivir is absorbed systemically (371). Absorbed oseltamivir is metabolized to oseltamivir carboxylate, the active neuraminidase inhibitor, primarily by hepatic esterases. Oseltamivir carboxylate has a half-life of 6--10 hours and is excreted in the urine by glomerular filtration and tubular secretion via the anionic pathway (310,373). Unmetabolized oseltamivir also is excreted in the urine by glomerular filtration and tubular secretion (325). Side Effects and Adverse ReactionsWhen considering use of influenza antiviral medications (i.e., choice of antiviral drug, dosage, and duration of therapy), clinicians must consider the patient's age, weight, and renal function (Table 6); presence of other medical conditions; indications for use (i.e., chemoprophylaxis or treatment); and the potential for interaction with other medications. Zanamivir In a study of zanamivir treatment of ILI among persons with asthma or chronic obstructive pulmonary disease where study medication was administered after use of a B2-agonist, 13% of patients receiving zanamivir and 14% of patients who received placebo (inhaled powdered lactose vehicle) experienced a >20% decline in forced expiratory volume in 1 second (FEV1) after treatment (317,330). However, in a phase I study of persons with mild or moderate asthma who did not have ILI, one of 13 patients experienced bronchospasm after administration of zanamivir (317). In addition, during postmarketing surveillance, cases of respiratory function deterioration after inhalation of zanamivir have been reported. Certain patients had underlying airway disease (e.g., asthma or chronic obstructive pulmonary disease). Because of the risk for serious adverse events and because the efficacy has not been demonstrated among this population, zanamivir is not recommended for treatment for patients with underlying airway disease (317). If physicians decide to prescribe zanamivir to patients with underlying chronic respiratory disease after carefully considering potential risks and benefits, the drug should be used with caution under conditions of appropriate monitoring and supportive care, including the availability of short-acting bronchodilators (355). Patients with asthma or chronic obstructive pulmonary disease who use zanamivir are advised to 1) have a fast-acting inhaled bronchodilator available when inhaling zanamivir and 2) stop using zanamivir and contact their physician if they experience difficulty breathing (317). No definitive evidence is available regarding the safety or efficacy of zanamivir for persons with underlying respiratory or cardiac disease or for persons with complications of acute influenza (355). Allergic reactions, including oropharyngeal or facial edema, also have been reported during postmarketing surveillance (317,337). In clinical treatment studies of persons with uncomplicated influenza, the frequencies of adverse events were similar for persons receiving inhaled zanamivir and for those receiving placebo (i.e., inhaled lactose vehicle alone) (320--325,337). The most common adverse events reported by both groups were diarrhea; nausea; sinusitis; nasal signs and symptoms; bronchitis; cough; headache; dizziness; and ear, nose, and throat infections. Each of these symptoms was reported by <5% of persons in the clinical treatment studies combined (317). Oseltamivir Nausea and vomiting were reported more frequently among adults receiving oseltamivir for treatment (nausea without vomiting, approximately 10%; vomiting, approximately 9%) than among persons receiving placebo (nausea without vomiting, approximately 6%; vomiting, approximately 3%) (310,326,327,374). Among children treated with oseltamivir, 14% had vomiting, compared with 8.5% of placebo recipients. Overall, 1% discontinued the drug secondary to this side effect (329), whereas a limited number of adults who were enrolled in clinical treatment trials of oseltamivir discontinued treatment because of these symptoms (310). Similar types and rates of adverse events were reported in studies of oseltamivir chemoprophylaxis (310). Nausea and vomiting might be less severe if oseltamivir is taken with food (317,310). Use During PregnancyNo clinical studies have been conducted regarding the safety or efficacy of zanamivir or oseltamivir for pregnant women. Because of the unknown effects of influenza antiviral drugs on pregnant women and their fetuses, these two drugs should be used during pregnancy only if the potential benefit justifies the potential risk to the embryo or fetus. Oseltamivir and zanamivir are both "Pregnancy Category C" medications (see manufacturers' package inserts) (317,375). Drug InteractionsClinical data are limited regarding drug interactions with zanamivir. However, no known drug interactions have been reported, and no clinically critical drug interactions have been predicted on the basis of in vitro data and data from studies using rats (310,373). Limited clinical data are available regarding drug interactions with oseltamivir. Because oseltamivir and oseltamivir carboxylate are excreted in the urine by glomerular filtration and tubular secretion via the anionic pathway, a potential exists for interaction with other agents excreted by this pathway. For example, coadministration of oseltamivir and probenecid resulted in reduced clearance of oseltamivir carboxylate by approximately 50% and a corresponding approximate twofold increase in the plasma levels of oseltamivir carboxylate (304,367). No published data are available concerning the safety or efficacy of using combinations of any of these influenza antiviral drugs. For more detailed information concerning potential drug interactions for any of these influenza antiviral drugs, package inserts should be consulted. Information Regarding the Vaccines for Children ProgramThe Vaccines for Children (VFC) program supplies vaccine to all states, territories, and the District of Columbia for use by participating providers. These vaccines are to be administered to eligible children without vaccine cost to the patient, as well as the provider. All routine childhood vaccines recommended by ACIP are available through this program. The program saves parents and providers out-of-pocket expenses for vaccine purchases and provides cost-savings to states through the CDC vaccine contracts. The program results in lower vaccine prices and assures that all states pay the same contract prices. Detailed information regarding the VFC program is available at http://www.cdc.gov/nip/vfc/default.htm. Sources of Information Regarding Influenza and Its SurveillanceInformation regarding influenza surveillance, prevention, detection, and control is available at http://www.cdc.gov/flu/weekly/fluactivity.htm. Surveillance information is available through the CDC Voice Information System (influenza update) at 888-232-3228 or CDC Fax Information Service at 888-232-3299. During October--May, surveillance information is updated weekly. In addition, periodic updates regarding influenza are published in the MMWR Weekly Report (http://www.cdc.gov/mmwr). Additional information regarding influenza vaccine can be obtained by calling 800-CDC-INFO (800-232-4636). State and local health departments should be consulted concerning availability of influenza vaccine, access to vaccination programs, information related to state or local influenza activity, reporting of influenza outbreaks and influenza-related pediatric deaths, and advice concerning outbreak control. Reporting of Adverse Events Following VaccinationClinically significant adverse events that follow vaccination should be reported through VAERS at http://vaers.hhs.gov or by calling the 24-hour national toll-free hotline at 800-822-7967. Additional Information Regarding Influenza Virus Infection Control Among Specific PopulationsEach year, ACIP provides general, annually updated information regarding control and prevention of influenza. Other reports related to controlling and preventing influenza among specific populations (e.g., immunocompromised persons, health-care workers, hospital patients, pregnant women, children, and travelers) also are available in the following publications:
References
* One dose equals 0.5 mL, divided equally between each nostril. These persons should receive inactivated influenza vaccine.
Advisory Committee on Immunization Practices Membership List, February 2006 Chairman: Jon Abramson, MD, Wake Forest University School of Medicine, Winston-Salem, North Carolina. Executive Secretary: Larry Pickering, MD, National Center for Immunization and Respiratory Diseases (proposed), Centers for Disease Control and Prevention, Atlanta, Georgia. Members: Ban Mishu Allos, MD, Vanderbilt University School of Medicine, Nashville, Tennessee; Robert Beck, Consumer Representative, Palmyra, Virginia; Judith Campbell, MD, Baylor College of Medicine, Houston, Texas; Reginald Finger, MD, Focus on the Family, Colorado Springs, Colorado; Janet Gildsdorf, MD, University of Michigan, Ann Arbor, Michigan; Harry Hull, MD, Minnesota Department of Health, Minneapolis, Minnesota; Tracy Lieu, MD, Harvard Pilgrim Health Care and Harvard Medical School, Boston, Massachusetts; Edgar Marcuse, MD, Children's Hospital and Regional Medical Center, Seattle, Washington; Dale Morse, MD, New York State Department of Health, Albany, New York; Julia Morita, MD, Chicago Department of Health, Chicago, Illinois; Gregory Poland, MD, Mayo Clinic College of Medicine, Rochester, Minnesota; Patricia Stinchfield, NP, Children's Hospital and Clinics, St. Paul, Minnesota; John J. Treanor, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York; and Robin Womeodu, MD, University of Tennessee Health Sciences Center, Memphis, Tennessee. Ex-Officio Members: James E. Cheek, MD, Indian Health Service, Albuquerque, New Mexico; Wayne Hachey, DO, Department of Defense, Falls Church, Virginia; Geoffrey S. Evans, MD, Health Resources and Services Administration, Rockville, Maryland; Bruce Gellin, MD, National Vaccine Program Office, Washington, DC; Linda Murphy, Centers for Medicare and Medicaid Services, Baltimore, Maryland; George T. Curlin, MD, National Institutes of Health, Bethesda, Maryland; Norman Baylor, MD, Food and Drug Administration, Bethesda, Maryland; Kristin Lee Nichol, MD, Department of Veterans Affairs, Minneapolis, Minnesota. Liaison Representatives: American Academy of Family Physicians, Jonathan Temte, MD, Clarence, New York, and Richard Clover, MD, Louisville, Kentucky; American Academy of Pediatrics, Keith Powell, MD, and Carol Baker, MD, Houston, Texas; American Association of Health Plans, Andrea Gelzer, MD, Hartford, Connecticut; American College Health Association, James C. Turner, MD, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Stanley Gall, MD, Louisville, Kentucky; American College of Physicians, Kathleen M. Neuzil, MD, Seattle, Washington; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Teachers of Preventive Medicine, W. Paul McKinney, MD, Louisville, Kentucky; Biotechnology Industry Organization, Clement Lewin, PhD, Cambridge, Massachusetts; Canadian National Advisory Committee on Immunization, Monica Naus, MD, Vancouver, British Columbia; Healthcare Infection Control Practices Advisory Committee, Steve Gordon, MD, Cleveland, Ohio; Infectious Diseases Society of America, Samuel L. Katz, MD, Durham, North Carolina, and William Schaffner, MD, Nashville, Tennessee; London Department of Health, David M. Salisbury, MD, London, United Kingdom; National Association of County and City Health Officials, Nancy Bennett, MD, Rochester, New York; National Coalition for Adult Immunization, David A. Neumann, PhD, Bethesda, Maryland; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Immunization Council and Child Health Program, Mexico, Romeo S. Rodriguez, Mexico City, Mexico; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick, New Jersey; National Vaccine Advisory Committee, Charles Helms, MD, PhD, Iowa City, Iowa; Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania, and Peter Paradiso, PhD, Collegeville, Pennsylvania; and Society for Adolescent Medicine, Amy Middleman, MD, Houston, Texas.
ACIP Influenza Working Group Chair: Ban Mishu Allos, MD, Nashville, Tennessee. Members: Jon Abramson, MD, Winston-Salem, North Carolina; Nancy Bennett, MD, Rochester, New York; Henry Bernstein, DO, Lebanon, New Hampshire; Joseph Bresee, MD, Atlanta, Georgia; Angela Calugar, MD, Atlanta, GA; Richard Clover, MD, Louisville, Kentucky; Nancy Cox, PhD, Atlanta, Georgia; Therese Cvetkovich, MD, Rockville, Maryland; Kathryn Edwards, MD, Nashville, Tennessee; Stanley Gall, MD, Louisville, Kentucky; Antonia Geber, MD, Rockville, Maryland; Penina Haber, MPH, Atlanta, Georgia; Guillermo Herrera, MD, Atlanta, Georgia; Harry Hull, MD, St. Paul, Minnesota; Marika Iwane, PhD, Atlanta, Georgia; Jim LeDuc, PhD, Atlanta, Georgia; Susan Lett, MD, Boston, Massachusetts; Roland Levandowski, MD, Bethesda, Maryland; Alison Mawle, PhD, Atlanta, Georgia; Kathleen Neuzil, MD, Seattle, Washington; Kristin Lee Nichol, MD, Minneapolis, Minnesota; William Schaffner, MD, Nashville, Tennessee; Benjamin Schwartz, MD, Atlanta, Georgia; David Shay, MD, Atlanta, Georgia; Nicole Smith, PhD, Atlanta, Georgia; Ray Strikas, MD, Atlanta, Georgia; Litjen Tan, PhD, Chicago, Illinois; John Treanor, MD, Rochester, New York; and Greg Wallace, MD, Atlanta, Georgia. Table 1 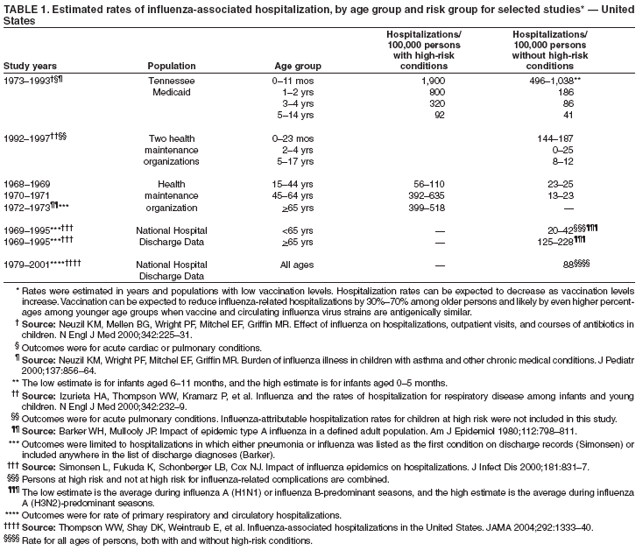 Return to top. Table 2 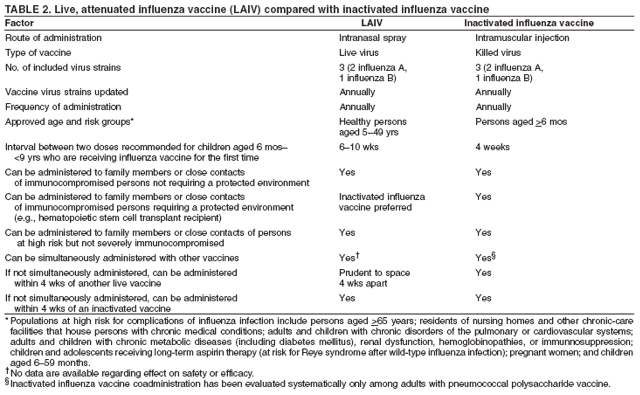 Return to top. Table 3 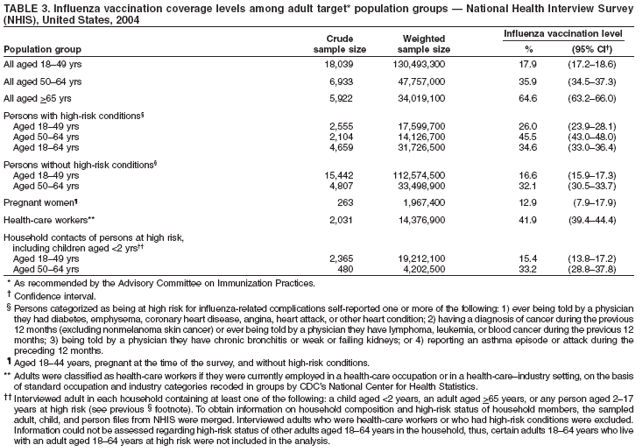 Return to top. Table 4 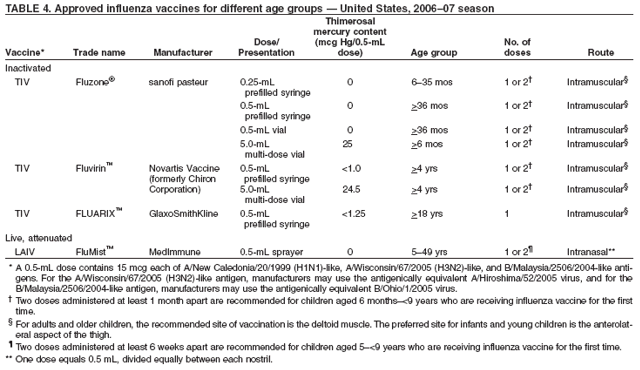 Return to top. Table 5  Return to top. Table 6 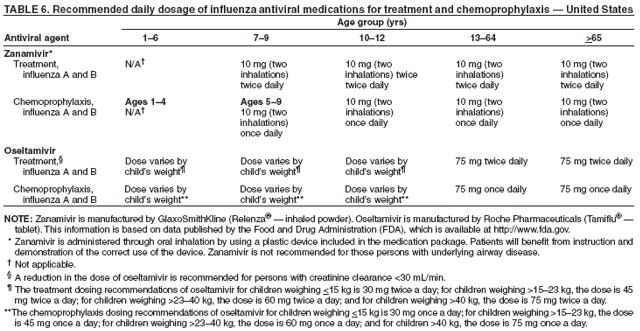 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 6/28/2006 |
|||||||||
|