 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure ProphylaxisSummaryThis report updates and consolidates all previous U.S. Public Health Service recommendations for the management of health-care personnel (HCP) who have occupational exposure to blood and other body fluids that might contain hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV). Recommendations for HBV postexposure management include initiation of the hepatitis B vaccine series to any susceptible, unvaccinated person who sustains an occupational blood or body fluid exposure. Postexposure prophylaxis (PEP) with hepatitis B immune globulin (HBIG) and/or hepatitis B vaccine series should be considered for occupational exposures after evaluation of the hepatitis B surface antigen status of the source and the vaccination and vaccine-response status of the exposed person. Guidance is provided to clinicians and exposed HCP for selecting the appropriate HBV PEP. Immune globulin and antiviral agents (e.g., interferon with or without ribavirin) are not recommended for PEP of hepatitis C. For HCV postexposure management, the HCV status of the source and the exposed person should be determined, and for HCP exposed to an HCV positive source, follow-up HCV testing should be performed to determine if infection develops. Recommendations for HIV PEP include a basic 4-week regimen of two drugs (zidovudine [ZDV] and lamivudine [3TC]; 3TC and stavudine [d4T]; or didanosine [ddI] and d4T) for most HIV exposures and an expanded regimen that includes the addition of a third drug for HIV exposures that pose an increased risk for transmission. When the source person's virus is known or suspected to be resistant to one or more of the drugs considered for the PEP regimen, the selection of drugs to which the source person's virus is unlikely to be resistant is recommended. In addition, this report outlines several special circumstances (e.g., delayed exposure report, unknown source person, pregnancy in the exposed person, resistance of the source virus to antiretroviral agents, or toxicity of the PEP regimen) when consultation with local experts and/or the National Clinicians' Post-Exposure Prophylaxis Hotline ([PEPline] 1-888-448-4911) is advised. Occupational exposures should be considered urgent medical concerns to ensure timely postexposure management and administration of HBIG, hepatitis B vaccine, and/or HIV PEP.
INTRODUCTIONAvoiding occupational blood exposures is the primary way to prevent transmission of hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in health-care settings (1). However, hepatitis B immunization and postexposure management are integral components of a complete program to prevent infection following bloodborne pathogen exposure and are important elements of workplace safety (2). The U.S. Public Health Service (PHS) has published previous guidelines for the management of HIV exposures that included considerations for postexposure prophylaxis (PEP) (3--5). Since publication of the 1998 HIV exposure guidelines (5), several new antiretroviral agents have been approved by the Food and Drug Administration (FDA), and more information is available about the use and safety of HIV PEP (6--11). In addition, questions exist regarding considerations about PEP regimens when the source person's virus is known or suspected to be resistant to one or more of the antiretroviral agents that might be used for PEP. Concern also has arisen about the use of PEP when it is not warranted. Data indicate that some health-care personnel (HCP) take a full course of HIV PEP after exposures that do not confer an HIV transmission risk (10,11). In September 1999, a meeting of a PHS interagency working group* and expert consultants was convened by CDC. The PHS working group decided to issue updated recommendations for the management of occupational exposure to HIV. In addition, the report was to include recommendations for the management of occupational HBV and HCV exposures so that a single document could comprehensively address the management of occupational exposures to bloodborne pathogens. This report updates and consolidates the previous PHS guidelines and recommendations for occupational HBV, HCV, and HIV exposure management for HCP. Specific practice recommendations for the management of occupational bloodborne pathogen exposures are outlined to assist health-care institutions with the implementation of these PHS guidelines (Appendices A and B). As relevant information becomes available, updates of these recommendations will be published. Recommendations for nonoccupational (e.g., sexual, pediatric, and perinatal) HBV, HCV, and HIV exposures are not addressed in these guidelines and can be found elsewhere (12--15). Definition of Health-Care Personnel and Exposure In this report, health-care personnel (HCP) are defined as persons (e.g., employees, students, contractors, attending clinicians, public-safety workers, or volunteers) whose activities involve contact with patients or with blood or other body fluids from patients in a health-care, laboratory, or public-safety setting. The potential exists for blood and body fluid exposure to other workers, and the same principles of exposure management could be applied to other settings. An exposure that might place HCP at risk for HBV, HCV, or HIV infection is defined as a percutaneous injury (e.g., a needlestick or cut with a sharp object) or contact of mucous membrane or nonintact skin (e.g., exposed skin that is chapped, abraded, or afflicted with dermatitis) with blood, tissue, or other body fluids that are potentially infectious (16,17). In addition to blood and body fluids containing visible blood, semen and vaginal secretions also are considered potentially infectious. Although semen and vaginal secretions have been implicated in the sexual transmission of HBV, HCV, and HIV, they have not been implicated in occupational transmission from patients to HCP. The following fluids also are considered potentially infectious: cerebrospinal fluid, synovial fluid, pleural fluid, peritoneal fluid, pericardial fluid, and amniotic fluid. The risk for transmission of HBV, HCV, and HIV infection from these fluids is unknown; the potential risk to HCP from occupational exposures has not been assessed by epidemiologic studies in health-care settings. Feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are not considered potentially infectious unless they contain blood. The risk for transmission of HBV, HCV, and HIV infection from these fluids and materials is extremely low. Any direct contact (i.e., contact without barrier protection) to concentrated virus in a research laboratory or production facility is considered an exposure that requires clinical evaluation. For human bites, the clinical evaluation must include the possibility that both the person bitten and the person who inflicted the bite were exposed to bloodborne pathogens. Transmission of HBV or HIV infection only rarely has been reported by this route (18--20) (CDC, unpublished data, 1998). BACKGROUNDThis section provides the rationale for the postexposure management and prophylaxis recommendations presented in this report. Additional details concerning the risk for occupational bloodborne pathogen transmission to HCP and management of occupational bloodborne pathogen exposures are available elsewhere (5,12,13,21-24). Occupational Transmission of HBV Risk for Occupational Transmission of HBV HBV infection is a well recognized occupational risk for HCP (25). The risk of HBV infection is primarily related to the degree of contact with blood in the work place and also to the hepatitis B e antigen (HBeAg) status of the source person. In studies of HCP who sustained injuries from needles contaminated with blood containing HBV, the risk of developing clinical hepatitis if the blood was both hepatitis B surface antigen (HBsAg)- and HBeAg-positive was 22%--31%; the risk of developing serologic evidence of HBV infection was 37%--62%. By comparison, the risk of developing clinical hepatitis from a needle contaminated with HBsAg-positive, HBeAg-negative blood was 1%--6%, and the risk of developing serologic evidence of HBV infection, 23%--37% (26). Although percutaneous injuries are among the most efficient modes of HBV transmission, these exposures probably account for only a minority of HBV infections among HCP. In several investigations of nosocomial hepatitis B outbreaks, most infected HCP could not recall an overt percutaneous injury (27,28), although in some studies, up to one third of infected HCP recalled caring for a patient who was HBsAg-positive (29,30). In addition, HBV has been demonstrated to survive in dried blood at room temperature on environmental surfaces for at least 1 week (31). Thus, HBV infections that occur in HCP with no history of nonoccupational exposure or occupational percutaneous injury might have resulted from direct or indirect blood or body fluid exposures that inoculated HBV into cutaneous scratches, abrasions, burns, other lesions, or on mucosal surfaces (32--34). The potential for HBV transmission through contact with environmental surfaces has been demonstrated in investigations of HBV outbreaks among patients and staff of hemodialysis units (35--37). Blood contains the highest HBV titers of all body fluids and is the most important vehicle of transmission in the health-care setting. HBsAg is also found in several other body fluids, including breast milk, bile, cerebrospinal fluid, feces, nasopharyngeal washings, saliva, semen, sweat, and synovial fluid (38). However, the concentration of HBsAg in body fluids can be 100--1000---fold higher than the concentration of infectious HBV particles. Therefore, most body fluids are not efficient vehicles of transmission because they contain low quantities of infectious HBV, despite the presence of HBsAg. In serologic studies conducted in the United States during the 1970s, HCP had a prevalence of HBV infection approximately 10 times higher than the general population (39--42). Because of the high risk of HBV infection among HCP, routine preexposure vaccination of HCP against hepatitis B and the use of standard precautions to prevent exposure to blood and other potentially infectious body fluids have been recommended since the early 1980s (43). Regulations issued by the Occupational Safety and Health Administration (OSHA) (2) have increased compliance with these recommendations. Since the implementation of these recommendations, a sharp decline has occurred in the incidence of HBV infection among HCP. PEP for HBV Efficacy of PEP for HBV. The effectiveness of hepatitis B immune globulin (HBIG) and/or hepatitis B vaccine in various postexposure settings has been evaluated by prospective studies. For perinatal exposure to an HBsAg-, HBeAg-positive mother, a regimen combining HBIG and initiation of the hepatitis B vaccine series at birth is 85%--95% effective in preventing HBV infection (44,45). Regimens involving either multiple doses of HBIG alone or the hepatitis B vaccine series alone are 70%--75% effective in preventing HBV infection (46). In the occupational setting, multiple doses of HBIG initiated within 1 week following percutaneous exposure to HBsAg-positive blood provides an estimated 75% protection from HBV infection (47--49). Although the postexposure efficacy of the combination of HBIG and the hepatitis B vaccine series has not been evaluated in the occupational setting, the increased efficacy of this regimen observed in the perinatal setting, compared with HBIG alone, is presumed to apply to the occupational setting as well. In addition, because persons requiring PEP in the occupational setting are generally at continued risk for HBV exposure, they should receive the hepatitis B vaccine series. Safety of PEP for HBV. Hepatitis B vaccines have been found to be safe when administered to infants, children, or adults (12,50). Through the year 2000, approximately 100 million persons have received hepatitis B vaccine in the United States. The most common side effects from hepatitis B vaccination are pain at the injection site and mild to moderate fever (50--55). Studies indicate that these side effects are reported no more frequently among persons vaccinated than among those receiving placebo (51,52). Approximately 45 reports have been received by the Vaccine Adverse Event Reporting System (VAERS) of alopecia (hair loss) in children and adults after administration of plasma-derived and recombinant hepatitis B vaccine; four persons sustained hair loss following vaccination on more than one occasion (56). Hair loss was temporary for approximately two thirds of persons who experienced hair loss. An epidemiologic study conducted in the Vaccine Safety Datalink found no statistical association between alopecia and receipt of hepatitis B vaccine in children (CDC, unpublished data, 1998). A low rate of anaphylaxis has been observed in vaccine recipients based on reports to VAERS; the estimated incidence is 1 in 600,000 vaccine doses distributed. Although none of the persons who developed anaphylaxis died, anaphylactic reactions can be life-threatening; therefore, further vaccination with hepatitis B vaccine is contraindicated in persons with a history of anaphylaxis after a previous dose of vaccine. Hepatitis B immunization programs conducted on a large scale in Taiwan, Alaska, and New Zealand have observed no association between vaccination and the occurrence of serious adverse events. Furthermore, in the United States, surveillance of adverse events following hepatitis B vaccination has demonstrated no association between hepatitis B vaccine and the occurrence of serious adverse events, including Guillain-Barré syndrome, transverse myelitis, multiple sclerosis, optic neuritis, and seizures (57--59) (CDC, unpublished data, 1991). However, several case reports and case series have claimed an association between hepatitis B vaccination and such syndromes and diseases as multiple sclerosis, optic neuritis, rheumatoid arthritis, and other autoimmune diseases (57,60--66). Most of these reported adverse events have occurred in adults, and no report has compared the frequency of the purported vaccine-associated syndrome/disease with the frequency in an unvaccinated population. In addition, recent case-control studies have demonstrated no association between hepatitis B vaccination and development or short-term risk of relapse of multiple sclerosis (67,68), and reviews by international panels of experts have concluded that available data do not demonstrate a causal association between hepatitis B vaccination and demyelinating diseases, including multiple sclerosis (69). HBIG is prepared from human plasma known to contain a high titer of antibody to HBsAg (anti-HBs). The plasma from which HBIG is prepared is screened for HBsAg and antibodies to HIV and HCV. The process used to prepare HBIG inactivates and eliminates HIV from the final product. Since 1996, the final product has been free of HCV RNA as determined by the polymerase chain reaction (PCR), and, since 1999, all products available in the United States have been manufactured by methods that inactivate HCV and other viruses. No evidence exists that HBV, HCV, or HIV have ever been transmitted by HBIG commercially available in the United States (70,71). Serious adverse effects from HBIG when administered as recommended have been rare. Local pain and tenderness at the injection site, urticaria and angioedema might occur; anaphylactic reactions, although rare, have been reported following the injection of human immune globulin (IG) preparations (72). Persons with a history of anaphylactic reaction to IG should not receive HBIG. PEP for HBV During Pregnancy. No apparent risk exists for adverse effects to developing fetuses when hepatitis B vaccine is administered to pregnant women (CDC, unpublished data, 1990). The vaccine contains noninfectious HBsAg particles and should pose no risk to the fetus. HBV infection during pregnancy might result in severe disease for the mother and chronic infection for the newborn. Therefore, neither pregnancy nor lactation should be considered a contraindication to vaccination of women. HBIG is not contraindicated for pregnant or lactating women. Occupational Transmission of HCV Risk for Occupational Transmission of HCV HCV is not transmitted efficiently through occupational exposures to blood. The average incidence of anti-HCV seroconversion after accidental percutaneous exposure from an HCV-positive source is 1.8% (range: 0%--7%) (73--76), with one study indicating that transmission occurred only from hollow-bore needles compared with other sharps (75). Transmission rarely occurs from mucous membrane exposures to blood, and no transmission in HCP has been documented from intact or nonintact skin exposures to blood (77,78). Data are limited on survival of HCV in the environment. In contrast to HBV, the epidemiologic data for HCV suggest that environmental contamination with blood containing HCV is not a significant risk for transmission in the health-care setting (79,80), with the possible exception of the hemodialysis setting where HCV transmission related to environmental contamination and poor infection-control practices have been implicated (81--84). The risk for transmission from exposure to fluids or tissues other than HCV-infected blood also has not been quantified but is expected to be low. Postexposure Management for HCV In several studies, researchers have attempted to assess the effectiveness of IG following possible exposure to non-A, non-B hepatitis. These studies have been difficult to interpret because they lack uniformity in diagnostic criteria and study design, and, in all but one study, the first dose of IG was administered before potential exposure (48,85,86). In an experiment designed to model HCV transmission by needlestick exposure in the health-care setting, high anti-HCV titer IG administered to chimpanzees 1 hour after exposure to HCV-positive blood did not prevent transmission of infection (87). In 1994, the Advisory Committee on Immunization Practices (ACIP) reviewed available data regarding the prevention of HCV infection with IG and concluded that using IG as PEP for hepatitis C was not supported (88). This conclusion was based on the following facts:
No clinical trials have been conducted to assess postexposure use of antiviral agents (e.g., interferon with or without ribavirin) to prevent HCV infection, and antivirals are not FDA-approved for this indication. Available data suggest that an established infection might need to be present before interferon can be an effective treatment. Kinetic studies suggest that the effect of interferon on chronic HCV infection occurs in two phases. During the first phase, interferon blocks the production or release of virus from infected cells. In the second phase, virus is eradicated from the infected cells (89); in this later phase, higher pretreatment alanine aminotransferase (ALT) levels correlate with an increasing decline in infected cells, and the rapidity of the decline correlates with viral clearance. In contrast, the effect of antiretrovirals when used for PEP after exposure to HIV is based on inhibition of HIV DNA synthesis early in the retroviral replicative cycle. In the absence of PEP for HCV, recommendations for postexposure management are intended to achieve early identification of chronic disease and, if present, referral for evaluation of treatment options. However, a theoretical argument is that intervention with antivirals when HCV RNA first becomes detectable might prevent the development of chronic infection. Data from studies conducted outside the United States suggest that a short course of interferon started early in the course of acute hepatitis C is associated with a higher rate of resolved infection than that achieved when therapy is begun after chronic hepatitis C has been well established (90--92). These studies used various treatment regimens and included persons with acute disease whose peak ALT levels were 500--1,000 IU/L at the time therapy was initiated (2.6--4 months after exposure). No studies have evaluated the treatment of acute infection in persons with no evidence of liver disease (i.e., HCV RNA-positive <6 months duration with normal ALT levels); among patients with chronic HCV infection, the efficacy of antivirals has been demonstrated only among patients who also had evidence of chronic liver disease (i.e., abnormal ALT levels). In addition, treatment started early in the course of chronic HCV infection (i.e., 6 months after onset of infection) might be as effective as treatment started during acute infection (13). Because 15%--25% of patients with acute HCV infection spontaneously resolve their infection (93), treatment of these patients during the acute phase could expose them unnecessarily to the discomfort and side effects of antiviral therapy. Data upon which to base a recommendation for therapy of acute infection are insufficient because a) no data exist regarding the effect of treating patients with acute infection who have no evidence of disease, b) treatment started early in the course of chronic infection might be just as effective and would eliminate the need to treat persons who will spontaneously resolve their infection, and c) the appropriate regimen is unknown. Occupational Transmission of HIV Risk for Occupational Transmission of HIV In prospective studies of HCP, the average risk of HIV transmission after a percutaneous exposure to HIV-infected blood has been estimated to be approximately 0.3% (95% confidence interval [CI] = 0.2%--0.5%) (94) and after a mucous membrane exposure, approximately 0.09% (95% CI = 0.006%--0.5%) (95). Although episodes of HIV transmission after nonintact skin exposure have been documented (96), the average risk for transmission by this route has not been precisely quantified but is estimated to be less than the risk for mucous membrane exposures (97). The risk for transmission after exposure to fluids or tissues other than HIV-infected blood also has not been quantified but is probably considerably lower than for blood exposures (98). As of June 2000, CDC had received voluntary reports of 56 U.S. HCP with documented HIV seroconversion temporally associated with an occupational HIV exposure. An additional 138 episodes in HCP are considered possible occupational HIV transmissions. These workers had a history of occupational exposure to blood, other infectious body fluids, or laboratory solutions containing HIV, and no other risk for HIV infection was identified, but HIV seroconversion after a specific exposure was not documented (99). Epidemiologic and laboratory studies suggest that several factors might affect the risk of HIV transmission after an occupational exposure. In a retrospective case-control study of HCP who had percutaneous exposure to HIV, the risk for HIV infection was found to be increased with exposure to a larger quantity of blood from the source person as indicated by a) a device visibly contaminated with the patient's blood, b) a procedure that involved a needle being placed directly in a vein or artery, or c) a deep injury (100). The risk also was increased for exposure to blood from source persons with terminal illness, possibly reflecting either the higher titer of HIV in blood late in the course of AIDS or other factors (e.g., the presence of syncytia-inducing strains of HIV). A laboratory study that demonstrated that more blood is transferred by deeper injuries and hollow-bore needles lends further support for the observed variation in risk related to blood quantity (101). The use of source person viral load as a surrogate measure of viral titer for assessing transmission risk has not yet been established. Plasma viral load (e.g., HIV RNA) reflects only the level of cell-free virus in the peripheral blood; latently infected cells might transmit infection in the absence of viremia. Although a lower viral load (e.g., <1,500 RNA copies/mL) or one that is below the limits of detection probably indicates a lower titer exposure, it does not rule out the possibility of transmission. Some evidence exists regarding host defenses possibly influencing the risk for HIV infection. A study of HIV-exposed but uninfected HCP demonstrated an HIV-specific cytotoxic T-lymphocyte (CTL) response when peripheral blood mononuclear cells were stimulated in vitro with HIV-specific antigens (102). Similar CTL responses have been observed in other groups who experienced repeated HIV exposure without resulting infection (103--108). Among several possible explanations for this observation is that the host immune response sometimes might prevent establishment of HIV infection after a percutaneous exposure; another is that the CTL response simply might be a marker for exposure. In a study of 20 HCP with occupational exposure to HIV, a comparison was made of HCP treated with zidovudine (ZDV) PEP and those not treated. The findings from this study suggest that ZDV blunted the HIV-specific CTL response and that PEP might inhibit early HIV replication (109). Rationale for HIV PEP Considerations that influence the rationale and recommendations for PEP include
The following discussion considers each of these concerns. Role of Pathogenesis in Considering Antiretroviral Prophylaxis. Information about primary HIV infection indicates that systemic infection does not occur immediately, leaving a brief window of opportunity during which postexposure antiretroviral intervention might modify or prevent viral replication. In a primate model of simian immunodeficiency virus (SIV) infection, infection of dendritic-like cells occurred at the site of inoculation during the first 24 hours following mucosal exposure to cell-free virus. Over the subsequent 24--48 hours, migration of these cells to regional lymph nodes occurred, and virus was detectable in the peripheral blood within 5 days (110). Theoretically, initiation of antiretroviral PEP soon after exposure might prevent or inhibit systemic infection by limiting the proliferation of virus in the initial target cells or lymph nodes. Efficacy of Antiretrovirals for PEP in Animal Studies. Data from animal studies have been difficult to interpret, in part, because of problems identifying an animal model that is comparable to humans. In early studies, differences in controlled variables (e.g., choice of viral strain [based on the animal model used], inoculum size, route of inoculation, time of prophylaxis initiation, and drug regimen) made extrapolation of the results to humans difficult. Recently, refinements in methodology have facilitated more relevant studies; in particular, the viral inocula used in animal studies have been reduced to levels more analogous to human exposures but sufficient to cause infection in control animals (111--113). These studies provide encouraging evidence of postexposure chemoprophylactic efficacy. Studies among primates and in murine and feline animal models have demonstrated that larger viral inocula decrease prophylactic efficacy (114--117). In addition, delaying initiation, shortening the duration, or decreasing the antiretroviral dose of PEP, individually or in combination, decreased prophylactic efficacy (113,118--124). For example, when (R)-9-(2-phosphonylmethoxypropyl) adenine (tenofovir) was administered 48 hours before, 4 hours after, or 24 hours after intravenous SIV inoculation to long-tailed macaques, a 4-week regimen prevented infection in all treated animals (122). A subsequent study confirmed the efficacy of tenofovir PEP when administered 24 hours after intravenous inoculation of a dose of SIV that uniformly results in infection in untreated macaques. In the same study, protection was incomplete if the tenofovir administration was delayed to 48 or 72 hours postexposure or if the total duration of treatment was curtailed to 3 or 10 days (123). Efficacy of Antiretrovirals for PEP in Human Studies. Little information exists from which the efficacy of PEP in humans can be assessed. Seroconversion is infrequent following an occupational exposure to HIV-infected blood; therefore, several thousands of exposed HCP would need to enroll in a prospective trial to achieve the statistical power necessary to directly demonstrate PEP efficacy (125). In the retrospective case-control study of HCP, after controlling for other risk factors for HIV transmission, use of ZDV as PEP was associated with a reduction in the risk of HIV infection by approximately 81% (95% CI = 43%--94%) (100). Although the results of this study suggest PEP efficacy, its limitations include the small number of cases studied and the use of cases and controls from different cohorts. In a multicenter trial in which ZDV was administered to HIV-infected pregnant women and their infants, the administration of ZDV during pregnancy, labor, and delivery and to the infant reduced transmission by 67% (126). Only part of the protective effect of ZDV was explained by reduction of the HIV viral load in the maternal blood, suggesting that ZDV prophylaxis, in part, involves a mechanism other than the reduction of maternal viral burden (127,128). Since 1998, studies have highlighted the importance of PEP for prevention of perinatal HIV transmission. In Africa, the use of ZDV in combination with lamivudine (3TC) decreased perinatal HIV transmission by 50% when administered during pregnancy, labor, and for 1 week postpartum, and by 37% when started at the onset of labor and continued for 1 week postpartum (129). Studies in the United States and Uganda also have demonstrated that rates of perinatal HIV transmission have been reduced with the use of abbreviated PEP regimens started intrapartum or during the first 48--72 hours of life (130--132). The limitations of all of these studies with animals and humans must be considered when reviewing evidence of PEP efficacy. The extent to which data from animal studies can be extrapolated to humans is largely unknown, and the exposure route for mother-to-infant HIV transmission is not similar to occupational exposures; therefore, these findings might not be directly applicable to PEP in HCP. Reports of Failure of PEP. Failure of PEP to prevent HIV infection in HCP has been reported in at least 21 instances (78,133--139). In 16 of the cases, ZDV was used alone as a single agent; in two cases, ZDV and didanosine (ddI) were used in combination (133,138); and in three cases, >3 drugs were used for PEP (137--139). Thirteen of the source persons were known to have been treated with antiretroviral therapy before the exposure. Antiretroviral resistance testing of the virus from the source person was performed in seven instances, and in four, the HIV infection transmitted was found to have decreased sensitivity to ZDV and/or other drugs used for PEP. In addition to possible exposure to an antiretroviral-resistant strain of HIV, other factors that might have contributed to these apparent failures might include a high titer and/or large inoculum exposure, delayed initiation and/or short duration of PEP, and possible factors related to the host (e.g., cellular immune system responsiveness) and/or to the source person's virus (e.g., presence of syncytia-forming strains) (133). Details regarding the cases of PEP failure involving combinations of antiretroviral agents are included in this report (Table 1). Antiretroviral Agents for PEP Antiretroviral agents from three classes of drugs are available for the treatment of HIV infection. These agents include the nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs). Only antiretroviral agents that have been approved by FDA for treatment of HIV infection are discussed in these guidelines. Determining which agents and how many to use or when to alter a PEP regimen is largely empiric. Guidelines for the treatment of HIV infection, a condition usually involving a high total body burden of HIV, include recommendations for the use of three drugs (140); however, the applicability of these recommendations to PEP remains unknown. In HIV-infected patients, combination regimens have proved superior to monotherapy regimens in reducing HIV viral load, reducing the incidence of opportunistic infections and death, and delaying onset of drug resistance (141,142). A combination of drugs with activity at different stages in the viral replication cycle (e.g., nucleoside analogues with a PI) theoretically could offer an additional preventive effect in PEP, particularly for occupational exposures that pose an increased risk of transmission. Although the use of a three-drug regimen might be justified for exposures that pose an increased risk of transmission, whether the potential added toxicity of a third drug is justified for lower-risk exposures is uncertain. Therefore, the recommendations at the end of this document provide guidance for two- and three-drug PEP regimens that are based on the level of risk for HIV transmission represented by the exposure. NRTI combinations that can be considered for PEP include ZDV and 3TC, 3TC and stavudine (d4T), and ddI and d4T. In previous PHS guidelines, a combination of ZDV and 3TC was considered the first choice for PEP regimens (3). Because ZDV and 3TC are available in a combination formulation (Combivir, manufactured by Glaxo Wellcome, Inc., Research Triangle Park, NC), the use of this combination might be more convenient for HCP. However, recent data suggest that mutations associated with ZDV and 3TC resistance might be common in some areas (143). Thus, individual clinicians might prefer other NRTIs or combinations based on local knowledge and experience in treating HIV infection and disease. The addition of a third drug for PEP following high-risk exposures is based on demonstrated effectiveness in reducing viral burden in HIV-infected persons. Previously, indinavir (IDV) or nelfinavir (NFV) were recommended as first-choice agents for inclusion in an expanded PEP regimen (5). Since the publication of the 1998 PEP guidelines, efavirenz (EFV), an NNRTI; abacavir (ABC), a potent NRTI; and Kaletra, a PI, have been approved by FDA. Although side effects might be common with the NNRTIs, EFV might be considered for expanded PEP regimens, especially when resistance to PIs in the source person's virus is known or suspected. ABC has been associated with dangerous hypersensitivity reactions but, with careful monitoring, may be considered as a third drug for PEP. Kaletra, a combination of lopinavir and ritonavir, is a potent HIV inhibitor that, with expert consultation, may be considered in an expanded PEP regimen. Toxicity and Drug Interactions of Antiretroviral Agents. When administering PEP, an important goal is completion of a 4-week PEP regimen when PEP is indicated. Therefore, the toxicity profile of antiretroviral agents, including the frequency, severity, duration, and reversibility of side effects, is a relevant consideration. All of the antiretroviral agents have been associated with side effects (Table 2). However, studies of adverse events have been conducted primarily with persons who have advanced disease (and longer treatment courses) and who therefore might not reflect the experience in persons who are uninfected (144). Several primary side effects are associated with antiretroviral agents (Table 2). Side effects associated with many of the NRTIs are chiefly gastrointestinal (e.g., nausea or diarrhea); however, ddI has been associated with cases of fatal and nonfatal pancreatitis among HIV-infected patients treated for >4 weeks. The use of PIs has been associated with new onset diabetes mellitus, hyperglycemia, diabetic ketoacidosis, exacerbation of preexisting diabetes mellitus, and dyslipidemia (145--147). Nephrolithiasis has been associated with IDV use; however, the incidence of this potential complication might be limited by drinking at least 48 ounces (1.5 L) of fluid per 24-hour period (e.g., six 8- ounce glasses of water throughout the day) (148). NFV has been associated with the development of diarrhea; however, this side effect might respond to treatment with antimotility agents that can be prescribed for use, if necessary, at the time the drug is recommended for PEP. The NNRTIs have been associated with severe skin reactions, including life-threatening cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Hepatotoxicity, including fatal hepatic necrosis, has occurred in patients treated with nevirapine (NVP); some episodes began during the first few weeks of therapy (FDA, unpublished data, 2000). EFV has been associated with central nervous system side effects, including dizziness, somnolence, insomnia, and abnormal dreaming. All of the approved antiretroviral agents might have potentially serious drug interactions when used with certain other drugs (Appendix C). Careful evaluation of concomitant medications used by an exposed person is required before PEP is prescribed, and close monitoring for toxicity is also needed. Further information about potential drug interactions can be found in the manufacturer's package insert. Toxicity Associated with PEP. Information from the National Surveillance System for Health Care Workers (NaSH) and the HIV Postexposure Registry indicates that nearly 50% of HCP experience adverse symptoms (e.g., nausea, malaise, headache, anorexia, and headache) while taking PEP and that approximately 33% stop taking PEP because of adverse signs and symptoms (6,7,10,11). Some studies have demonstrated that side effects and discontinuation of PEP are more common among HCP taking three-drug combination regimens for PEP compared with HCP taking two-drug combination regimens (7,10). Although similar rates of side effects were observed among persons who took PEP after sexual or drug use exposures to HIV in the San Francisco Post-Exposure Prevention Project, 80% completed 4 weeks of therapy (149). Participants in the San Francisco Project were followed at 1, 2, 4, 26, and 52 weeks postexposure and received medication adherence counseling; most participants took only two drugs for PEP. Serious side effects, including nephrolithiasis, hepatitis, and pancytopenia have been reported with the use of combination drugs for PEP (6,7,150,151). One case of NVP-associated fulminant liver failure requiring liver transplantation and one case of hypersensitivity syndrome have been reported in HCP taking NVP for HIV PEP (152). Including these two cases, from March 1997 through September 2000, FDA received reports of 22 cases of serious adverse events related to NVP taken for PEP (153). These events included 12 cases of hepatotoxicity, 14 cases of skin reaction (including one documented and two possible cases of Stevens-Johnson syndrome), and one case of rhabdomyolysis; four cases involved both hepatotoxicty and skin reaction, and one case involved both rhabdomyolysis and skin reaction. Resistance to Antiretroviral Agents. Known or suspected resistance of the source virus to antiretroviral agents, particularly to agents that might be included in a PEP regimen, is a concern for persons making decisions about PEP. Resistance to HIV infection occurs with all of the available antiretroviral agents, and cross-resistance within drug classes is frequent (154). Recent studies have demonstrated an emergence of drug-resistant HIV among source persons for occupational exposures (143,155). A study conducted at seven U.S. sites during 1998--1999 found that 16 (39%) of 41 source persons whose virus was sequenced had primary genetic mutations associated with resistance to RTIs, and 4 (10%) had primary mutations associated with resistance to PIs (143). In addition, occupational transmission of resistant HIV strains, despite PEP with combination drug regimens, has been reported (137,139). In one case, a hospital worker became infected after an HIV exposure despite a PEP regimen that included ddI, d4T, and NVP (139). The transmitted HIV contained two primary genetic mutations associated with resistance to NNRTIs (the source person was taking EFV at the time of the exposure). Despite recent studies and case reports, the relevance of exposure to a resistant virus is still not well understood. Empiric decisions about the presence of antiretroviral drug resistance are often difficult to make because patients generally take more than one antiretroviral agent. Resistance should be suspected in source persons when they are experiencing clinical progression of disease or a persistently increasing viral load, and/or decline in CD4 T-cell count, despite therapy or a lack of virologic response to therapy. However, resistance testing of the source virus at the time of an exposure is not practical because the results will not be available in time to influence the choice of the initial PEP regimen. Furthermore, in this situation, whether modification of the PEP regimen is necessary or will influence the outcome of an occupational exposure is unknown. No data exist to suggest that modification of a PEP regimen after receiving results from resistance testing (usually a minimum of 1--2 weeks) improves efficacy of PEP. Antiretroviral Drugs During Pregnancy. Data are limited on the potential effects of antiretroviral drugs on the developing fetus or neonate (156). Carcinogenicity and/or mutagenicity is evident in several in vitro screening tests for ZDV and all other FDA-licensed NRTIs. The relevance of animal data to humans is unknown; however, because teratogenic effects were observed in primates at drug exposures similar to those representing human therapeutic exposure, the use of EFV should be avoided in pregnant women (140). IDV is associated with infrequent side effects in adults (i.e., hyperbilirubinemia and renal stones) that could be problematic for a newborn. Because the half-life of IDV in adults is short, these concerns might be relevant only if the drug is administered shortly before delivery. In a recent study in France of perinatal HIV transmission, two cases of progressive neurologic disease and death were reported in uninfected infants exposed to ZDV and 3TC (157). Laboratory studies of these children suggested mitochondrial dysfunction. In a careful review of deaths in children followed in U.S. perinatal HIV cohorts, no deaths attributable to mitochondrial disease have been found (158). Recent reports of fatal and nonfatal lactic acidosis in pregnant women treated throughout gestation with a combination of d4T and ddI have prompted warnings about use of these drugs during pregnancy (159). Although the case-patients were HIV-infected women taking the drugs for >4 weeks, pregnant women and their providers should be advised to consider d4T and ddI only when the benefits of their use outweigh the risks. PEP Use in Hospitals in the United States. Analysis of data from NaSH provides information on the use of PEP following occupational exposures in 47 hospitals in the United States. A total of 11,784 exposures to blood and body fluids was reported from June 1996 through November 2000 (CDC, unpublished data, 2001). For all exposures with known sources, 6% were to HIV-positive sources, 74% to HIV-negative sources, and 20% to sources with an unknown HIV status. Sixty-three percent of HCP exposed to a known HIV-positive source started PEP, and 54% of HCP took it for at least 20 days, whereas 14% of HCP exposed to a source person subsequently found to be HIV-negative initiated PEP, and 3% of those took it for at least 20 days. Information recorded about HIV exposures in NaSH indicates that 46% of exposures involving an HIV-positive source warranted only a two-drug PEP regimen (i.e., the exposure was to mucous membranes or skin or was a superficial percutaneous injury and the source person did not have end-stage AIDS or acute HIV illness); however, 53% of these exposed HCP took >3 drugs (CDC, unpublished data, 2000). Similarly, the National Clinicians' Post-Exposure Prophylaxis Hotline (PEPline) reported that PEPline staff recommended stopping or not starting PEP for approximately one half of the HCP who consulted them about exposures (D. Bangsberg, San Francisco General Hospital, unpublished data, September 1999). The observation that some HCP exposed to HIV-negative source persons take PEP from several days to weeks following their exposures suggests that strategies be employed such as the use of a rapid HIV antibody assay, which could minimize exposure to unnecessary PEP (11). A recent study demonstrated that use of a rapid HIV test for evaluation of source persons after occupational exposures not only resulted in decreased use of PEP, but also was cost-effective compared with use of the standard enzyme immunoassay (EIA) test for source persons subsequently found to be HIV-negative (160). RECOMMENDATIONS FOR THE MANAGEMENT OF HCP POTENTIALLY EXPOSED TO HBV, HCV, or HIVExposure prevention remains the primary strategy for reducing occupational bloodborne pathogen infections; however, occupational exposures will continue to occur. Health-care organizations should make available to their personnel a system that includes written protocols for prompt reporting, evaluation, counseling, treatment, and follow-up of occupational exposures that might place HCP at risk for acquiring a bloodborne infection. HCP should be educated concerning the risk for and prevention of bloodborne infections, including the need to be vaccinated against hepatitis B (17,21,161--163). Employers are required to establish exposure-control plans that include postexposure follow-up for their employees and to comply with incident reporting requirements mandated by the 1992 OSHA bloodborne pathogen standard (2). Access to clinicians who can provide postexposure care should be available during all working hours, including nights and weekends. HBIG, hepatitis B vaccine, and antiretroviral agents for HIV PEP should be available for timely administration (i.e., either by providing access on-site or by creating linkages with other facilities or providers to make them available off-site). Persons responsible for providing postexposure management should be familiar with evaluation and treatment protocols and the facility's plans for accessing HBIG, hepatitis B vaccine, and antiretroviral drugs for HIV PEP. HCP should be educated to report occupational exposures immediately after they occur, particularly because HBIG, hepatitis B vaccine, and HIV PEP are most likely to be effective if administered as soon after the exposure as possible. HCP who are at risk for occupational exposure to bloodborne pathogens should be familiarized with the principles of postexposure management as part of job orientation and ongoing job training. Hepatitis B Vaccination Any person who performs tasks involving contact with blood, blood-contaminated body fluids, other body fluids, or sharps should be vaccinated against hepatitis B (2,21). Prevaccination serologic screening for previous infection is not indicated for persons being vaccinated because of occupational risk, unless the hospital or health-care organization considers screening cost-effective. Hepatitis B vaccine should always be administered by the intramuscular route in the deltoid muscle with a needle 1--1.5 inches long. Hepatitis B vaccine can be administered at the same time as other vaccines with no interference with antibody response to the other vaccines (164). If the vaccination series is interrupted after the first dose, the second dose should be administered as soon as possible. The second and third doses should be separated by an interval of at least 2 months. If only the third dose is delayed, it should be administered when convenient. HCP who have contact with patients or blood and are at ongoing risk for percutaneous injuries should be tested 1--2 months after completion of the 3dose vaccination series for anti-HBs (21). Persons who do not respond to the primary vaccine series (i.e., anti-HBs <10 mIU/mL) should complete a second 3-dose vaccine series or be evaluated to determine if they are HBsAg-positive. Revaccinated persons should be retested at the completion of the second vaccine series. Persons who do not respond to an initial 3-dose vaccine series have a 30%--50% chance of responding to a second 3-dose series (165). Persons who prove to be HBsAg-positive should be counseled regarding how to prevent HBV transmission to others and regarding the need for medical evaluation (12,163,166). Nonresponders to vaccination who are HBsAg-negative should be considered susceptible to HBV infection and should be counseled regarding precautions to prevent HBV infection and the need to obtain HBIG prophylaxis for any known or probable parenteral exposure to HBsAg-positive blood. Booster doses of hepatitis B vaccine are not necessary, and periodic serologic testing to monitor antibody concentrations after completion of the vaccine series is not recommended. Any blood or body fluid exposure sustained by an unvaccinated, susceptible person should lead to the initiation of the hepatitis B vaccine series. Treatment of an Exposure Site Wounds and skin sites that have been in contact with blood or body fluids should be washed with soap and water; mucous membranes should be flushed with water. No evidence exists that using antiseptics for wound care or expressing fluid by squeezing the wound further reduces the risk of bloodborne pathogen transmission; however, the use of antiseptics is not contraindicated. The application of caustic agents (e.g., bleach) or the injection of antiseptics or disinfectants into the wound is not recommended. Exposure Report If an occupational exposure occurs, the circumstances and postexposure management should be recorded in the exposed person's confidential medical record (usually on a form the facility designates for this purpose) (Box 1). In addition, employers should follow all federal (including OSHA) and state requirements for recording and reporting occupational injuries and exposures. Evaluation of the Exposure and the Exposure Source Evaluation of the Exposure The exposure should be evaluated for the potential to transmit HBV, HCV, and HIV based on the type of body substance involved and the route and severity of the exposure (Box 2). Blood, fluid containing visible blood, or other potentially infectious fluid (including semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids) or tissue can be infectious for bloodborne viruses. Exposures to these fluids or tissue through a percutaneous injury (i.e., needlestick or other penetrating sharps-related event) or through contact with a mucous membrane are situations that pose a risk for bloodborne virus transmission and require further evaluation. For HCV and HIV, exposure to a blood-filled hollow needle or visibly bloody device suggests a higher risk exposure than exposure to a needle that was most likely used for giving an injection. In addition, any direct contact (i.e, personal protective equipment either was not present or was ineffective in protecting skin or mucous membranes) with concentrated virus in a research laboratory or production facility is considered an exposure that requires clinical evaluation. For skin exposure, follow-up is indicated only if it involves exposure to a body fluid previously listed and evidence exists of compromised skin integrity (e.g., dermatitis, abrasion, or open wound). In the clinical evaluation for human bites, possible exposure of both the person bitten and the person who inflicted the bite must be considered. If a bite results in blood exposure to either person involved, postexposure follow-up should be provided. Evaluation of the Exposure Source The person whose blood or body fluid is the source of an occupational exposure should be evaluated for HBV, HCV, and HIV infection (Box 3). Information available in the medical record at the time of exposure (e.g., laboratory test results, admitting diagnosis, or previous medical history) or from the source person, might confirm or exclude bloodborne virus infection. If the HBV, HCV, and/or HIV infection status of the source is unknown, the source person should be informed of the incident and tested for serologic evidence of bloodborne virus infection. Procedures should be followed for testing source persons, including obtaining informed consent, in accordance with applicable state and local laws. Any persons determined to be infected with HBV, HCV, or HIV should be referred for appropriate counseling and treatment. Confidentiality of the source person should be maintained at all times. Testing to determine the HBV, HCV, and HIV infection status of an exposure source should be performed as soon as possible. Hospitals, clinics and other sites that manage exposed HCP should consult their laboratories regarding the most appropriate test to use to expedite obtaining these results. An FDA-approved rapid HIV-antibody test kit should be considered for use in this situation, particularly if testing by EIA cannot be completed within 24--48 hours. Repeatedly reactive results by EIA or rapid HIV-antibody tests are considered to be highly suggestive of infection, whereas a negative result is an excellent indicator of the absence of HIV antibody. Confirmation of a reactive result by Western blot or immunofluorescent antibody is not necessary to make initial decisions about postexposure management but should be done to complete the testing process and before informing the source person. Repeatedly reactive results by EIA for anti-HCV should be confirmed by a supplemental test (i.e., recombinant immunoblot assay [RIBA] or HCV PCR). Direct virus assays (e.g., HIV p24 antigen EIA or tests for HIV RNA or HCV RNA) for routine HIV or HCV screening of source persons are not recommended. If the exposure source is unknown or cannot be tested, information about where and under what circumstances the exposure occurred should be assessed epidemiologically for the likelihood of transmission of HBV, HCV, or HIV. Certain situations as well as the type of exposure might suggest an increased or decreased risk; an important consideration is the prevalence of HBV, HCV, or HIV in the population group (i.e., institution or community) from which the contaminated source material is derived. For example, an exposure that occurs in a geographic area where injection-drug use is prevalent or involves a needle discarded in a drug-treatment facility would be considered epidemiologically to have a higher risk for transmission than an exposure that occurs in a nursing home for the elderly. Testing of needles or other sharp instruments implicated in an exposure, regardless of whether the source is known or unknown, is not recommended. The reliability and interpretation of findings in such circumstances are unknown, and testing might be hazardous to persons handling the sharp instrument. Examples of information to consider when evaluating an exposure source for possible HBV, HCV, or HIV infection include laboratory information (e.g., previous HBV, HCV, or HIV test results or results of immunologic testing [e.g., CD4+ T-cell count]) or liver enzymes (e.g., ALT), clinical symptoms (e.g., acute syndrome suggestive of primary HIV infection or undiagnosed immunodeficiency disease), and history of recent (i.e., within 3 months) possible HBV, HCV, or HIV exposures (e.g., injection-drug use or sexual contact with a known positive partner). Health-care providers should be aware of local and state laws governing the collection and release of HIV serostatus information on a source person, following an occupational exposure. If the source person is known to have HIV infection, available information about this person's stage of infection (i.e., asymptomatic, symptomatic, or AIDS), CD4+ T-cell count, results of viral load testing, current and previous antiretroviral therapy, and results of any genotypic or phenotypic viral resistance testing should be gathered for consideration in choosing an appropriate PEP regimen. If this information is not immediately available, initiation of PEP, if indicated, should not be delayed; changes in the PEP regimen can be made after PEP has been started, as appropriate. Reevaluation of exposed HCP should be considered within 72 hours postexposure, especially as additional information about the exposure or source person becomes available. If the source person is HIV seronegative and has no clinical evidence of AIDS or symptoms of HIV infection, no further testing of the person for HIV infection is indicated. The likelihood of the source person being in the "window period" of HIV infection in the absence of symptoms of acute retroviral syndrome is extremely small. Management of Exposures to HBV For percutaneous or mucosal exposures to blood, several factors must be considered when making a decision to provide prophylaxis, including the HBsAg status of the source and the hepatitis B vaccination and vaccine-response status of the exposed person. Such exposures usually involve persons for whom hepatitis B vaccination is recommended. Any blood or body fluid exposure to an unvaccinated person should lead to initiation of the hepatitis B vaccine series. The hepatitis B vaccination status and the vaccine-response status (if known) of the exposed person should be reviewed. A summary of prophylaxis recommendations for percutaneous or mucosal exposure to blood according to the HBsAg status of the exposure source and the vaccination and vaccine-response status of the exposed person is included in this report (Table 3). When HBIG is indicated, it should be administered as soon as possible after exposure (preferably within 24 hours). The effectiveness of HBIG when administered >7 days after exposure is unknown. When hepatitis B vaccine is indicated, it should also be administered as soon as possible (preferably within 24 hours) and can be administered simultaneously with HBIG at a separate site (vaccine should always be administered in the deltoid muscle). For exposed persons who are in the process of being vaccinated but have not completed the vaccination series, vaccination should be completed as scheduled, and HBIG should be added as indicated (Table 3). Persons exposed to HBsAg-positive blood or body fluids who are known not to have responded to a primary vaccine series should receive a single dose of HBIG and reinitiate the hepatitis B vaccine series with the first dose of the hepatitis B vaccine as soon as possible after exposure. Alternatively, they should receive two doses of HBIG, one dose as soon as possible after exposure, and the second dose 1 month later. The option of administering one dose of HBIG and reinitiating the vaccine series is preferred for nonresponders who did not complete a second 3-dose vaccine series. For persons who previously completed a second vaccine series but failed to respond, two doses of HBIG are preferred. Management of Exposures to HCV Individual institutions should establish policies and procedures for testing HCP for HCV after percutaneous or mucosal exposures to blood and ensure that all personnel are familiar with these policies and procedures. The following are recommendations for follow-up of occupational HCV exposures:
Health-care professionals who provide care to persons exposed to HCV in the occupational setting should be knowledgeable regarding the risk for HCV infection and appropriate counseling, testing, and medical follow-up. IG and antiviral agents are not recommended for PEP after exposure to HCV-positive blood. In addition, no guidelines exist for administration of therapy during the acute phase of HCV infection. However, limited data indicate that antiviral therapy might be beneficial when started early in the course of HCV infection. When HCV infection is identified early, the person should be referred for medical management to a specialist knowledgeable in this area. Counseling for HCP Exposed to Viral Hepatitis HCP exposed to HBV- or HCV-infected blood do not need to take any special precautions to prevent secondary transmission during the follow-up period (12,13); however, they should refrain from donating blood, plasma, organs, tissue, or semen. The exposed person does not need to modify sexual practices or refrain from becoming pregnant. If an exposed woman is breast feeding, she does not need to discontinue. No modifications to an exposed person's patient-care responsibilities are necessary to prevent transmission to patients based solely on exposure to HBV- or HCV-positive blood. If an exposed person becomes acutely infected with HBV, the person should be evaluated according to published recommendations for infected HCP (165). No recommendations exist regarding restricting the professional activities of HCP with HCV infection (13). As recommended for all HCP, those who are chronically infected with HBV or HCV should follow all recommended infection-control practices, including standard precautions and appropriate use of hand washing, protective barriers, and care in the use and disposal of needles and other sharp instruments (162). Management of Exposures to HIV Clinical Evaluation and Baseline Testing of Exposed HCP HCP exposed to HIV should be evaluated within hours (rather than days) after their exposure and should be tested for HIV at baseline (i.e., to establish infection status at the time of exposure). If the source person is seronegative for HIV, baseline testing or further follow-up of the exposed person normally is not necessary. Serologic testing should be made available to all HCP who are concerned that they might have been occupationally infected with HIV. For purposes of considering HIV PEP, the evaluation also should include information about medications the exposed person might be taking and any current or underlying medical conditions or circumstances (i.e., pregnancy, breast feeding, or renal or hepatic disease) that might influence drug selection. PEP for HIV The following recommendations (Table 4 and Table 5) apply to situations when a person has been exposed to a source person with HIV infection or when information suggests the likelihood that the source person is HIV-infected. These recommendations are based on the risk for HIV infection after different types of exposure and on limited data regarding efficacy and toxicity of PEP. Because most occupational HIV exposures do not result in the transmission of HIV, potential toxicity must be carefully considered when prescribing PEP. To assist with the initial management of an HIV exposure, health-care facilities should have drugs for an initial PEP regimen selected and available for use. When possible, these recommendations should be implemented in consultation with persons who have expertise in antiretroviral therapy and HIV transmission (Box 4). Timing and Duration of PEP. PEP should be initiated as soon as possible. The interval within which PEP should be initiated for optimal efficacy is not known. Animal studies have demonstrated the importance of starting PEP soon after an exposure (111,112,118). If questions exist about which antiretroviral drugs to use or whether to use a basic or expanded regimen, starting the basic regimen immediately rather than delaying PEP administration is probably better. Although animal studies suggest that PEP probably is substantially less effective when started more than 24--36 hours postexposure (112,119,122), the interval after which no benefit is gained from PEP for humans is undefined. Therefore, if appropriate for the exposure, PEP should be started even when the interval since exposure exceeds 36 hours. Initiating therapy after a longer interval (e.g., 1 week) might be considered for exposures that represent an increased risk for transmission. The optimal duration of PEP is unknown. Because 4 weeks of ZDV appeared protective in occupational and animal studies (100,123), PEP probably should be administered for 4 weeks, if tolerated. Use of PEP When HIV Infection Status of Source Person is Unknown. If the source person's HIV infection status is unknown at the time of exposure, use of PEP should be decided on a case-by-case basis, after considering the type of exposure and the clinical and/or epidemiologic likelihood of HIV infection in the source (Table 4 and Table 5). If these considerations suggest a possibility for HIV transmission and HIV testing of the source person is pending, initiating a two-drug PEP regimen until laboratory results have been obtained and later modifying or discontinuing the regimen accordingly is reasonable. The following are recommendations regarding HIV postexposure prophylaxis:
PEP for Pregnant HCP. If the exposed person is pregnant, the evaluation of risk of infection and need for PEP should be approached as with any other person who has had an HIV exposure. However, the decision to use any antiretroviral drug during pregnancy should involve discussion between the woman and her health-care provider(s) regarding the potential benefits and risks to her and her fetus. Certain drugs should be avoided in pregnant women. Because teratogenic effects were observed in primate studies, EFV is not recommended during pregnancy. Reports of fatal lactic acidosis in pregnant women treated with a combination of d4T and ddI have prompted warnings about these drugs during pregnancy. Because of the risk of hyperbilirubinemia in newborns, IDV should not be administered to pregnant women shortly before delivery. Recommendations for the Selection of Drugs for HIV PEP Health-care providers must strive to balance the risk for infection against the potential toxicity of the agent(s) used when selecting a drug regimen for HIV PEP. Because PEP is potentially toxic, its use is not justified for exposures that pose a negligible risk for transmission (Table 4 and Table 5). Also, insufficient evidence exists to support recommending a three-drug regimen for all HIV exposures. Therefore, two regimens for PEP are provided (Appendix C): a "basic" two-drug regimen that should be appropriate for most HIV exposures and an "expanded" three-drug regimen that should be used for exposures that pose an increased risk for transmission (Table 4 and Table 5). When possible, the regimens should be implemented in consultation with persons who have expertise in antiretroviral treatment and HIV transmission. Most HIV exposures will warrant a two-drug regimen using two nucleoside analogues (e.g., ZDV and 3TC; or 3TC and d4T; or d4T and ddI). The addition of a third drug should be considered for exposures that pose an increased risk for transmission. Selection of the PEP regimen should consider the comparative risk represented by the exposure and information about the exposure source, including history of and response to antiretroviral therapy based on clinical response, CD4+ T-cell counts, viral load measurements, and current disease stage. When the source person's virus is known or suspected to be resistant to one or more of the drugs considered for the PEP regimen, the selection of drugs to which the source person's virus is unlikely to be resistant is recommended; expert consultation is advised. If this information is not immediately available, initiation of PEP, if indicated, should not be delayed; changes in the PEP regimen can be made after PEP has been started, as appropriate. Reevaluation of the exposed person should be considered within 72 hours postexposure, especially as additional information about the exposure or source person becomes available. Follow-up of HCP Exposed to HIV Postexposure Testing. HCP with occupational exposure to HIV should receive follow-up counseling, postexposure testing, and medical evaluation, regardless of whether they receive PEP. HIV-antibody testing should be performed for at least 6 months postexposure (e.g., at 6 weeks, 12 weeks, and 6 months). Extended HIV follow-up (e.g., for 12 months) is recommended for HCP who become infected with HCV following exposure to a source coinfected with HIV and HCV. Whether extended follow-up is indicated in other circumstances (e.g., exposure to a source coinfected with HIV and HCV in the absence of HCV seroconversion or for exposed persons with a medical history suggesting an impaired ability to develop an antibody response to acute infection) is unclear. Although rare instances of delayed HIV seroconversion have been reported (167,168), the infrequency of this occurrence does not warrant adding to the anxiety level of the exposed persons by routinely extending the duration of postexposure follow-up. However, this recommendation should not preclude a decision to extend follow-up in an individual situation based on the clinical judgement of the exposed person's health-care provider. HIV testing should be performed on any exposed person who has an illness that is compatible with an acute retroviral syndrome, regardless of the interval since exposure. When HIV infection is identified, the person should be referred to a specialist knowledgeable in the area of HIV treatment and counseling for medical management. HIV-antibody testing with EIA should be used to monitor for seroconversion. The routine use of direct virus assays (e.g., HIV p24 antigen EIA or tests for HIV RNA) to detect infection in exposed HCP generally is not recommended (169). The high rate of false-positive results of these tests in this setting could lead to unnecessary anxiety and/or treatment (170,171). Despite the ability of direct virus assays to detect HIV infection a few days earlier than EIA, the infrequency of occupational seroconversion and increased costs of these tests do not warrant their routine use in this setting.
Monitoring and Management of PEP Toxicity. If PEP is used, HCP should be monitored for drug toxicity by testing at baseline and again 2 weeks after starting PEP. The scope of testing should be based on medical conditions in the exposed person and the toxicity of drugs included in the PEP regimen. Minimally, lab monitoring for toxicity should include a complete blood count and renal and hepatic function tests. Monitoring for evidence of hyperglycemia should be included for HCP whose regimens include any PI; if the exposed person is receiving IDV, monitoring for crystalluria, hematuria, hemolytic anemia, and hepatitis also should be included. If toxicity is noted, modification of the regimen should be considered after expert consultation; further diagnostic studies may be indicated. Exposed HCP who choose to take PEP should be advised of the importance of completing the prescribed regimen. Information should be provided to HCP about potential drug interactions and the drugs that should not be taken with PEP, the side effects of the drugs that have been prescribed, measures to minimize these effects, and the methods of clinical monitoring for toxicity during the follow-up period. HCP should be advised that the evaluation of certain symptoms should not be delayed (e.g., rash, fever, back or abdominal pain, pain on urination or blood in the urine, or symptoms of hyperglycemia [increased thirst and/or frequent urination]). HCP who fail to complete the recommended regimen often do so because of the side effects they experience (e.g., nausea and diarrhea). These symptoms often can be managed with antimotility and antiemetic agents or other medications that target the specific symptoms without changing the regimen. In other situations, modifying the dose interval (i.e., administering a lower dose of drug more frequently throughout the day, as recommended by the manufacturer), might facilitate adherence to the regimen. Serious adverse events should be reported to FDA's MedWatch Program. Counseling and Education. Although HIV infection following an occupational exposure occurs infrequently, the emotional effect of an exposure often is substantial (172--174). In addition, HCP are given seemingly conflicting information. Although HCP are told that a low risk exists for HIV transmission, a 4-week regimen of PEP might be recommended, and they are asked to commit to behavioral measures (e.g., sexual abstinence or condom use) to prevent secondary transmission, all of which influence their lives for several weeks to months (172). Therefore, access to persons who are knowledgeable about occupational HIV transmission and who can deal with the many concerns an HIV exposure might generate for the exposed person is an important element of postexposure management. HIV-exposed HCP should be advised to use the following measures to prevent secondary transmission during the follow-up period, especially the first 6--12 weeks after the exposure when most HIV-infected persons are expected to seroconvert: exercise sexual abstinence or use condoms to prevent sexual transmission and to avoid pregnancy; and refrain from donating blood, plasma, organs, tissue, or semen. If an exposed woman is breast feeding, she should be counseled about the risk of HIV transmission through breast milk, and discontinuation of breast feeding should be considered, especially for high-risk exposures. Additionally, NRTIs are known to pass into breast milk, as is NVP; whether this also is true for the other approved antiretroviral drugs is unknown. The patient-care responsibilities of an exposed person do not need to be modified, based solely on an HIV exposure, to prevent transmission to patients. If HIV seroconversion is detected, the person should be evaluated according to published recommendations for infected HCP (175). Exposed HCP should be advised to seek medical evaluation for any acute illness that occurs during the follow-up period. Such an illness, particularly if characterized by fever, rash, myalgia, fatigue, malaise, or lymphadenopathy, might be indicative of acute HIV infection but also might be indicative of a drug reaction or another medical condition. For exposures for which PEP is considered appropriate, HCP should be informed that a) knowledge about the efficacy of drugs used for PEP is limited; b) experts recommend combination drug regimens because of increased potency and concerns about drug-resistant virus; c) data regarding toxicity of antiretroviral drugs in persons without HIV infection or in pregnant women are limited; d) although the short-term toxicity of antiretroviral drugs is usually limited, serious adverse events have occurred in persons taking PEP; and e) any or all drugs for PEP may be declined or stopped by the exposed person. HCP who experience HIV occupational exposures for which PEP is not recommended should be informed that the potential side effects and toxicity of taking PEP outweigh the negligible risk of transmission posed by the type of exposure. Guidelines for counseling and educating HCP with HIV exposure include
Occupational Exposure Management Resources Several resources are available that provide guidance to HCP regarding the management of occupational exposures. These resources include PEPline; the Needlestick! website; the Hepatitis Hotline; CDC (receives reports of occupationally acquired HIV infections and failures of PEP); the HIV Antiretroviral Pregnancy Registry; FDA (receives reports of unusual or severe toxicity to antiretroviral agents); and the HIV/AIDS Treatment Information Service (Box 5). *This interagency working group comprised representatives of CDC, the Food and Drug Administration (FDA), the Health Resources and Services Administration, and the National Institutes of Health. Information included in these recommendations may not represent FDA approval or approved labeling for the particular product or indications in question. Specifically, the terms "safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval. References
Table 1 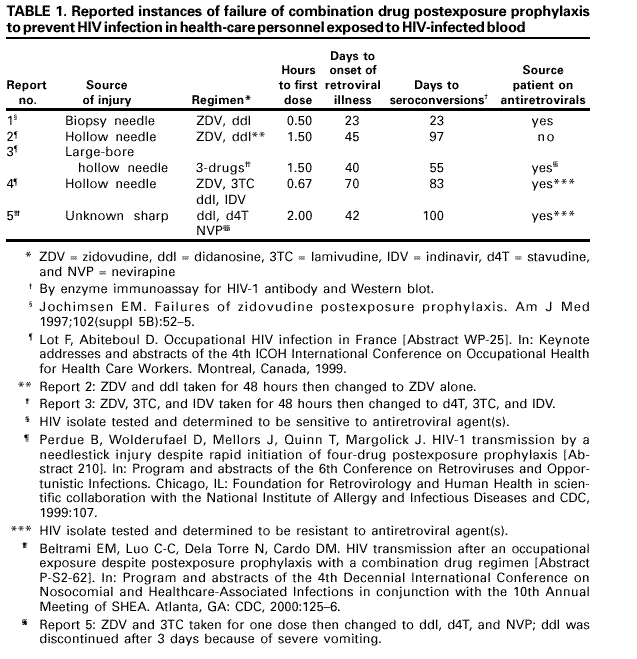 Return to top. Table 2 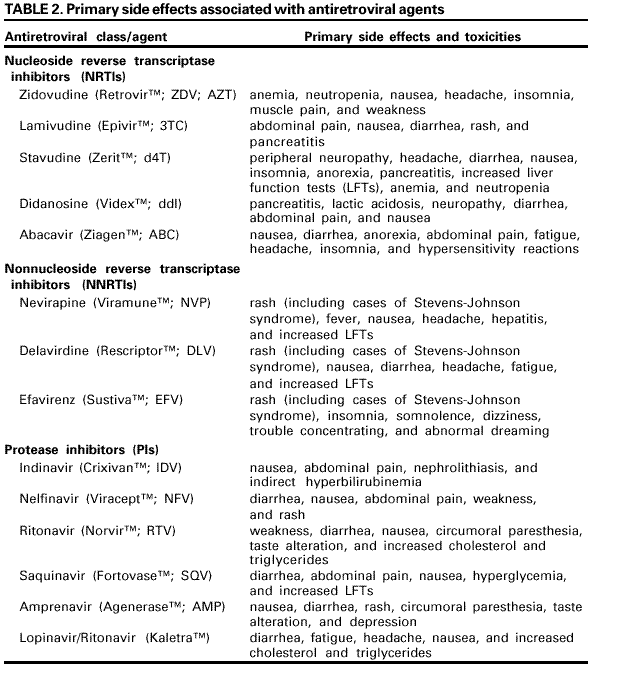 Return to top. Box 3 All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/2/2001 |
|||||||||
This page last reviewed 7/2/2001
|