 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Multistate Outbreak of Salmonella Infections Associated with Peanut Butter and Peanut Butter--Containing Products --- United States, 2008--2009On November 25, 2008, an epidemiologic assessment began of a growing cluster of Salmonella serotype Typhimurium isolates that shared the same pulsed-field gel electrophoresis (PFGE) pattern in PulseNet.* As of January 28, 2009, 529 persons from 43 states (Figure 1) and one person from Canada had been reported infected with the outbreak strain. This report is an interim summary of results from ongoing epidemiologic studies and recall and control activities by CDC, the Food and Drug Administration (FDA), and state and local public health agencies. Confirmed, reported onset of illness dates have ranged from September 1, 2008, to January 16, 2009. A total of 116 patients were reported hospitalized, and the infection might have contributed to eight deaths. Sequential case-control studies have indicated significant associations between illness and consumption of any peanut butter (matched odds ratio [mOR] = 2.53), and specific brands of prepackaged peanut butter crackers (mOR = 12.25), but no association with national brand jarred peanut butter sold in grocery stores. Epidemiologic and laboratory findings indicate that peanut butter and peanut paste produced at one plant are the source of the outbreak. These products also are ingredients in many foods produced and distributed by other companies. This outbreak highlights the complexities of "ingredient-driven" outbreaks and the importance of rapid outbreak detection and investigation. Consumers are advised to discard and not eat products that have been recalled (Box). Initial Outbreak InvestigationOn November 10, 2008, CDC's PulseNet staff noted a small and highly dispersed multistate cluster of 13 S. Typhimurium isolates with an unusual PFGE pattern (XbaI PFGE pattern JPXX01.1818) reported from 12 states. On November 25, CDC's OutbreakNet team, working with state and local partners, began an epidemiologic assessment of that cluster, which had increased to 35 isolates. On December 2, CDC and state and local partners began an assessment of a second cluster of 41 S. Typhimurium isolates. The PFGE patterns of the second cluster (XbaI pattern JPXX01.0459/JPXX01.1825) were very similar to the patterns in the first cluster and were first noted by PulseNet on November 24, as a cluster of 27 isolates that had subsequently increased to 41 isolates. None of these patterns were seen previously in the PulseNet S. Typhimurium database. Testing with a second PFGE enzyme (BlnI) showed that isolates from both clusters had the same pattern (JPXA26.0462) and were indistinguishable by multilocus variable-number tandem-repeat analysis, a different PulseNet subtyping method. The outbreak strain has the phage type 3 and is fully susceptible to all antimicrobials in the National Antibiotic Resistance Monitoring System panel for gram-negative bacteria. The clusters also appeared similar epidemiologically, so the two patterns were grouped together as a single outbreak strain, and the investigations were merged. The outbreak strain did not exist in the National VetNet database, which contains PFGE patterns of Salmonella isolates from raw meat and poultry products, and which CDC and the U.S. Department of Agriculture's Food Safety and Inspection Service monitor. A case was defined as a laboratory-confirmed infection of S. Typhimurium with the outbreak strain in a person with illness onset date (or, if that date was not known, with date of isolation of Salmonella) on or after September 1, 2008. As of January 28, 2009, onset dates were known for 424 of 529 patients and ranged from September 1, 2008, to January 16, 2009 (Figure 2). Although numbers of reported cases have decreased in recent weeks, the outbreak appears to be ongoing. The median age of patients was 16 years, with an age range of <1 to 98 years; 21% were aged <5 years, and 15% were aged >59 years. Of those patients, 48% were female, 116 (22%) were hospitalized, and the infection might have contributed to eight deaths in patients aged >59 years from Minnesota (three deaths), Virginia (two), Idaho (one), North Carolina (one), and Ohio (one). A median of 16 days elapsed from the day the illness began to the date the PFGE pattern was uploaded to PulseNet (Figure 2). The initial epidemiologic investigation included detailed open-ended interviews with patients. Patient interviews were conducted by CDC and state and local health departments using a questionnaire with approximately 300 food items. Early interviews, case reports, and identification of small clusters of cases suggested a possible association with institutional settings, although noninstitutionalized patients often reported consumption of peanut butter of multiple brands. In the initial investigation, among the most frequently reported food exposures in the 7 days before illness began, 86% of patients interviewed reported they were likely to have eaten chicken and 77% were likely to have eaten peanut butter. By comparison, the frequencies in the general public of eating these items were 85% for chicken and 59% for peanut butter in a 2006--2007 FoodNet§ food consumption survey (1). Association with Peanut ButterMany affected state health departments, including the Minnesota Department of Health (MDH), conducted intensive investigations of patients infected with the outbreak strain. By December 28, MDH had learned from patient interviews that some patients infected with the outbreak strain lived or ate meals in one of at least three institutions (two long-term--care facilities and one elementary school). A review of menus and invoices by MDH and the Minnesota Department of Agriculture (MDA) revealed that the institutions had a common food distributor in North Dakota, and the only food common to the three institutions was King Nut creamy peanut butter. By January 9, 2009, six additional cases in six other institutions were identified by MDH; each of those institutions had received King Nut peanut butter. An open container of King Nut peanut butter was collected from one of the institutions, a long-term--care facility, on January 5 for testing at MDA. On January 9, the MDA laboratory reported isolation of Salmonella from the King Nut peanut butter sample. This was confirmed on January 12 as S. Typhimurium of the outbreak strain. On January 3 and 4, 2009, data were gathered for a case-control study by CDC and state and local health departments to identify whether illness was associated with eating specific food items; 70 cases and 178 controls were enrolled from 12 participating states. For this study, a case was defined as infection with the outbreak strain of S. Typhimurium in a person without preceding diarrheal illness in household members and who did not live in an institutional setting, with illness onset (or, if that date was not known, with date of isolation of Salmonella) on or after November 1, 2008. Controls recruited using a reverse-digit--dialing system were well persons, matched by case neighborhood and age category (i.e., <18 years or >18 years). Food histories were sought for the 7 days before illness onset for case-patients and 7 days before interview for controls. The median ages for case-patients and controls were 18 and 16 years, respectively. By January 9, preliminary analysis found that case-patients were significantly more likely than controls to have eaten any peanut butter in the 7 days before illness began (69% of case-patients versus 48% of controls, mOR = 2.53, 95% confidence interval [CI] = 1.26--5.31, p=0.007). Illness also was associated with eating any of a group of previously frozen chicken products (i.e., chicken nuggets, chicken strips, and other breaded and stuffed chicken products) (35% of case-patients versus 14% of controls, mOR = 4.61, CI = 1.67--14.68, p=0.002), but not with any individual chicken product; no individual frozen chicken product type was reported eaten by more than 10% of case-patients. Illness was not associated with eating roasted peanuts or national brands of jarred peanut butter sold in grocery stores. On January 16, the Connecticut Department of Public Health Laboratory isolated the outbreak strain of S. Typhimurium from a previously unopened 5-pound container of King Nut creamy peanut butter. As of January 28, 16 clusters of cases, each with at least two patients infected with the outbreak strain, were reported in five states. All clusters were in institutional facilities. King Nut was the only brand of peanut butter used in the 16 facilities. All versions of King Nut peanut butter were produced by Peanut Corporation of America (PCA) at a single facility in Blakely, Georgia. An environmental investigation at the PCA plant was initiated by FDA and the Georgia Department of Agriculture on January 9, and a CDC epidemiologist joined the investigation team on January 10. King Nut peanut butter was distributed in bulk packaging to institutions, food service industries, and private label food companies. King Nut peanut butter was not known to be sold directly to consumers or distributed for retail sale in grocery stores. On January 22, MDA found that a previously unopened container of King Nut peanut butter collected from the North Dakota distributor yielded Salmonella serotype Tennessee with a PFGE pattern that was indistinguishable from an outbreak strain in the multistate outbreak in 2006--2007 caused by contaminated peanut butter (2). Association with Peanut Butter--Containing ProductsOngoing patient interviews indicated that many patients did not eat peanut butter in institutions, but had eaten various other peanut butter--containing products. FDA investigators reported that the PCA facility in Blakely produced peanut butter and also peanut paste (also made from ground roasted peanuts) and other peanut products, which were sold to many food companies for use as an ingredient in peanut butter--containing foods; these peanut butter--containing products are widely distributed in the United States and also are distributed in at least 23 other countries and non-U.S. territories.¶ During January 17--19, a second case-control study was conducted by CDC and state and local health departments to further assess these exposures; 93 cases and 399 controls were enrolled from 35 participating states. For this study, a case was defined as infection with the outbreak strain of S. Typhimurium in a person without preceding diarrheal illness in household members and who did not live in an institutional setting, with illness onset (or, if that date was not known, with date of isolation of Salmonella) on or after December 1, 2008. Controls were well persons, matched by case neighborhood and frequency matched by age groups (i.e., 0 to <6 years, 6 to <18 years, 18 to <40 years, and >40 years), who were recruited using a reverse-digit--dialing system. Controls were interviewed about the same exposure period as their matched case-patient (i.e., 7 days before the onset of the case diarrheal illness). Median ages of case-patients and controls were 17 and 39 years, respectively. Preliminary analysis found that patients were more likely than controls to have eaten prepackaged peanut butter crackers in the 7 days before illness began [73% case-patients versus 17% controls, mOR = 12.25, CI = 5.51--30.9, p<0.0001]. Two cracker brands were individually associated: Austin [43% case-patients versus 3% controls, mOR = 29.68, CI = 8.95--154.66, p<0.0001] and Keebler [20% case-patients versus 4% controls, mOR = 5.38, CI = 1.74--18.32, p=0.003] peanut butter crackers. Both Austin and Keebler brand peanut butter crackers are made at one plant, which is known to receive peanut paste from PCA. No evidence was discovered of an epidemiologic association with eating roasted peanuts. Intact packages of Austin brand Toasty peanut butter crackers that had been purchased in the United States were obtained from the home of a patient in Canada by the Canadian Food Inspection Agency. Culture of a composite sample of the crackers yielded the outbreak strain of S. Typhimurium. Salmonella resembling the outbreak strain was isolated by a private laboratory from three intact packages of Austin brand Toasty peanut butter crackers obtained from a patient's home in Oregon. Control MeasuresOn January 9, PCA voluntarily stopped production of peanut butter and peanut paste at the Blakely, Georgia, facility. On January 10, King Nut Company issued a voluntary recall of specific lot numbers of peanut butter manufactured by PCA and distributed under King Nut and Parnell's Pride labels. On January 16, PCA announced a voluntary recall of all peanut butter and peanut paste produced in its Blakely facility since July 1, 2008. On January 28, the PCA recall was expanded to include all peanuts and peanut products processed at this plant since January 1, 2007. In addition to peanut butter and peanut paste, the expanded recall includes dry- and oil-roasted peanuts, granulated peanuts, and peanut meal. On January 28, 2009, the facility reported that production of all peanut products had stopped. The latest information on the PCA recall can be found on the FDA website.** To date, FDA inspectors have traced the shipments of these products to approximately 2,100 accounts and sub-accounts. FDA is working to identify additional products that might be affected and to track the ingredient supply chain of those products to remove them from the marketplace. On January 14, the Kellogg Company announced a precautionary hold on Austin and Keebler brands of peanut butter crackers, and on January 16, voluntarily recalled these products produced after July 1, 2008. As of January 28, at least 431 peanut butter--containing products had been recalled by 54 companies that had used ingredients produced by the PCA facility after July 1, 2008. Reported by: C Medus, PhD, S Meyer, MPH, K Smith, DVM, Minnesota Dept of Health; S Jawahir, Minnesota Dept of Health Public Health Laboratory; B Miller, MPH, K Viger, MS, Minnesota Dept of Agriculture; M Forstner, Minnesota Dept of Agriculture Laboratory. E Brandt, S Nowicki, MPH, E Salehi, MPH, Ohio Dept of Health. Q Phan, MPH, A Kinney, M Cartter, MD, Connecticut Dept of Public Health. J Flint, MPH, Public Health Agency of Canada. J Bancroft, MPH, Oregon Public Health Div. J Adams, E Hyytia-Trees, PhD, L Theobald, D Talkington, PhD, M Humphrys, MS, C Bopp, MS, P Gerner-Smidt, MD, C Barton Behravesh, DVM, IT Williams, PhD, S Sodha, MD, A Langer, DVM, C Schwenson, MPH, F Angulo, DVM, Enteric Diseases Epidemiology Br; R Tauxe MD, Div of Bacterial, Foodborne and Mycotic Diseases; K Date, MD, E Cavallaro, MD, C Kim, PhD, EIS officers, CDC. Editorial Note:Each year, approximately 40,000 laboratory- confirmed cases of Salmonella infections are reported to the National Salmonella Surveillance System.§§ S. Typhimurium is the most commonly reported serotype. In 2006, 19% of all reported salmonellosis cases for which a serotype was identified were caused by the Typhimurium serotype (3). This outbreak likely is considerably larger than the 529 laboratory-confirmed cases reported to CDC; only an estimated 3% of Salmonella infections are laboratory confirmed and reported to surveillance systems (4). During 2003--2007, an annual average of 18 outbreaks caused by S. Typhimurium were reported to CDC.¶¶ The rates of hospitalization and mortality observed in the current outbreak are typical for Salmonella, and this strain does not appear to be unusually virulent. The epidemiologic and laboratory findings from this continuing investigation indicate that peanut butter and peanut paste produced at the PCA plant are the source of the outbreak. More specifically, the outbreak was caused by contaminated peanut butter used in institutions, and by peanut butter and peanut paste used as ingredients in food products. The second case- control study indicated a particular risk with peanut butter crackers, but this does not exonerate other peanut-containing products. After one brand of peanut butter served in institutions was implicated by epidemiologic and laboratory evidence, the investigation was expanded to include food items that use peanut butter and peanut paste made in the same factory as ingredients in peanut butter--containing products. This was an ingredient-driven outbreak, in which a contaminated ingredient affected many different products that are distributed through various channels and consumed in various settings. Peanut butter and peanut paste are common ingredients in cookies, crackers, cereal, candy, ice cream, pet treats, and other foods. Mass food distribution can lead to widely distributed nationwide outbreaks. The large number of products and brands recalled already, and the large quantities of some products recalled, makes this one of the largest recalls in the United States. This is the second outbreak caused by contaminated peanut butter in the United States. The first outbreak was caused by contamination of a commercially distributed brand of peanut butter with S. Tennessee during 2006--2007 (2). Only one other previous outbreak associated with peanut butter has been reported; an outbreak of Salmonella serotype Mbandaka infections in Australia in 1996 (5). The detection of a S. Tennessee isolate with a PFGE pattern that is indistinguishable from the 2006--2007 strain in a recently manufactured container of King Nut peanut butter is notable. However, the S. Tennessee strain is not associated with an increase in illnesses now. The implicated plant in 2006--2007 is located approximately 70 miles from the PCA plant in Blakely. A possible association between the two outbreaks warrants further investigation. The relationship of the S. Tennessee finding to the current outbreak is being investigated further. The mechanism of contamination for the current outbreak have not yet been determined. However, the recurring problem of Salmonella associated with contaminated peanut butter highlights the importance of including a kill step for harmful pathogens during manufacture (e.g., proper roasting) and of preventing contamination of peanut butter after the initial roasting process. Salmonella organisms persist indefinitely in high-fat, low-water-activity foods such as peanut butter (6), and in such foods, Salmonella can withstand temperatures as high as 194°F (90°C) for 50 minutes (7). Typically, peanuts for peanut butter are roasted at approximately 350°F (180°C), a temperature that should be sufficient to kill Salmonella in a short period. However, some temperatures used in processing peanut butter or paste in other products might be inadequate to eliminate Salmonella introduced after the initial peanut roasting. When this outbreak was first detected, its source was not immediately apparent. A likely source of the current outbreak emerged only after several weeks of detailed case interviews, investigations of local clusters of illness, and joint epidemiologic efforts across states. Rapid traceback of the first implicated product to its point of manufacture was critical in unraveling the entire outbreak. Rapid investigation of apparently localized outbreaks can provide critical clues to solving large and dispersed national outbreaks. This outbreak illustrates again the central importance of the capacity to perform Salmonella serotyping and molecular subtyping in public health laboratories for detecting and investigating outbreaks, and the critical value of rapid epidemiologic and regulatory investigative capacity. Acknowledgments This report is based, in part, on contributions by the Food and Drug Admin; F Greene, MPH, Connecticut Dept of Public Health Laboratory; WE Keene, PhD, HA Booth, Oregon Public Health Div; and RM Hoekstra, PhD, K Wannemuehler, PhD, Div of Bacterial, Foodborne and Mycotic Diseases, and volunteers in the Director's Emergency Operations Center, CDC References
* The national molecular subtyping network for foodborne disease surveillance. Includes amikacin, amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim-sulfphamethoxazole. § Foodborne diseases active surveillance network. ¶ As of January 27, 2009, FDA was aware of distribution in the following countries and non-U.S. territories: Aruba, Australia, the Bahamas, Bermuda, Canada, the Cayman Islands, Haiti, Italy, Jamaica, Japan, Korea, Malaysia, Mexico, the Netherlands, New Zealand, Norway, St. Maarten, St. Vincent and the Grenadines, Singapore, Slovenia, Spain, the Turks and Caicos Islands, and the United Kingdom.. ** Information on the PCA recall is available from FDA at http://www.fda.gov/oc/po/firmrecalls/peanutcorp401_09.html and at http://www.fda.gov/oc/opacom/hottopics/salmonellatyph.html. The latest update on the epidemiologic investigation is available at http://www.cdc.gov/salmonella/typhimurium. The current list of recalled products with a searchable format can be found on the FDA website (http://www.accessdata.fda.gov/scripts/peanutbutterrecall/index.cfm). §§ The National Salmonella Surveillance System collects information on serotypes of Salmonella isolates reported through the Public Health Laboratory Information System, an electronic reporting system. Additional information is available at http://www.cdc.gov/ncidod/dbmd/phlisdata/salmonella.htm. ¶¶ Data from CDC's Electronic Foodborne Outbreak Reporting System (eFORS), unpublished data; 2008. Cases reported as of January 29, 2009. Cases beginning in the most recent 3 weeks might not yet be reported. Figure 1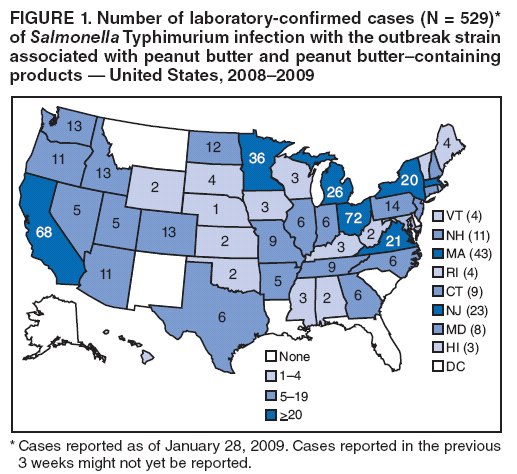 Return to top. Figure 2 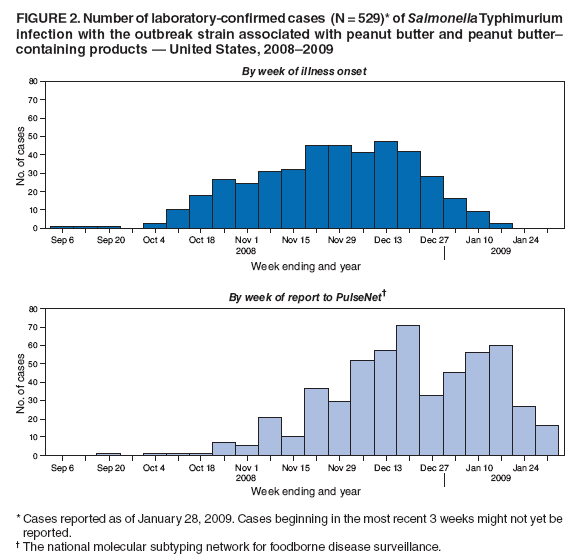 Return to top. Box 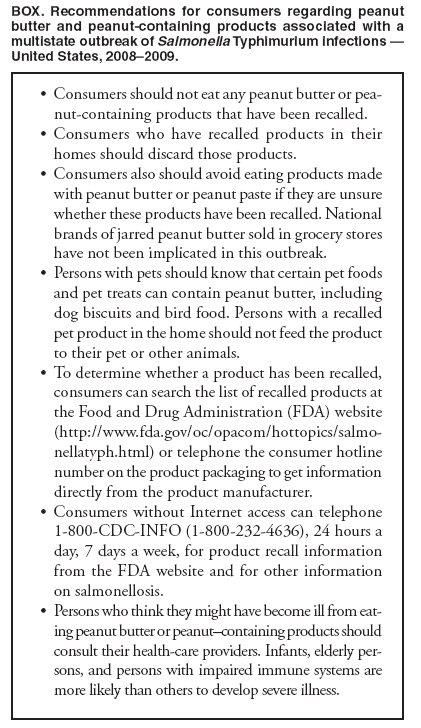 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 1/29/2009 |
|||||||||
|