 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hepatitis C Virus Transmission at an Outpatient Hemodialysis Unit --- New York, 2001--2008In July 2008, the New York State Department of Health (NYSDOH) received reports of three hemodialysis patients seroconverting from anti-hepatitis C virus (HCV) negative to anti-HCV positive in a New York City hemodialysis unit during the preceding 6 months. NYSDOH conducted patient interviews and made multiple visits to the hemodialysis unit to observe hemodialysis treatments, assess infection control practices, evaluate HCV surveillance activities, review medical records, and conduct interviews with staff members. This report summarizes the results of that investigation, which found that six additional patients had HCV seroconversion during 2001--2008 and that the hemodialysis unit had numerous deficiencies in infection control policies, procedures, and training. Of the total of nine seroconversions, the sources for four HCV infections were identified phylogenetically and epidemiologically as four other patients in the unit. The unit's policy for routine patient testing for HCV infection was not in accordance with CDC recommendations, and the few recommendations followed were not implemented consistently. Hemodialysis units should routinely assess compliance to ensure complete and timely adherence with CDC recommendations to reduce the risk for HCV transmission in this setting. The hemodialysis unit was a large, for-profit, outpatient facility treating 70--100 patients daily at 30 dialysis stations. On May 24, 2008, the New York City Department of Health and Mental Hygiene informed NYSDOH of a confirmed HCV seroconversion in one patient receiving chronic hemodialysis treatment at the unit. On July 1, the unit reported two additional HCV seroconversions directly to NYSDOH. Interviews conducted by NYSDOH with the three patients who seroconverted revealed no other common health-care exposures or behavioral risk factors. In addition, none of the three had been informed by the hemodialysis unit of their HCV infections. Initial site visit findings by NYSDOH documented poor infection control practices and oversight. Specific recommendations addressing deficiencies were provided to the unit's administrative staff members at the initial site visit and throughout the investigation. An epidemiologic investigation subsequently was undertaken to identify additional patients with HCV infection, assess infection control practices, and make recommendations to prevent ongoing transmission. Epidemiologic InvestigationThe epidemiologic study population consisted of all 162 patients who were receiving hemodialysis at the unit as of July 1, 2008. For all patients, HCV-related test results reported through the unit's central electronic laboratory system and the NYSDOH Electronic Clinical Laboratory Reporting System were reviewed, and patients were matched against New York state and New York City hepatitis surveillance registries. All current patients were offered anti-HCV testing. Because hemodialysis unit staff members were not considered likely sources of HCV transmission in this investigation, staff members were not tested. Patients were considered HCV positive if their serum was 1) determined positive by enzyme immunoassay (EIA) testing with a signal-to-cutoff ratio consistent with CDC recommendations for a confirmed anti-HCV positive test or 2) determined positive by EIA followed by recombinant immunoblot assay or nucleic acid testing for HCV RNA (1). A chronic case of HCV was defined as a case in a patient who was HCV positive before or upon admission to the hemodialysis unit. An incident case was defined as a case in a patient who was HCV negative upon admission to the hemodialysis unit but who subsequently was confirmed HCV positive. Unit medical records for all HCV-positive patients were reviewed, and serum from available patients was submitted to NYSDOH's Wadsworth Center laboratory for HCV sequencing and phylogenetic analysis. Of the 162 patients, HCV infection status at hemodialysis unit admission could be documented through medical records and previous test results for 110 (68%). Twenty (18%) of the 110 had chronic HCV infection at admission. Ninety (82%) were anti-HCV negative at admission, of whom nine (10%) were determined to have acquired incident HCV infection, seroconverting to anti-HCV positive during 2001--2008 (Figure). Among the 162 patients, a total of 45 (28%) had at least one anti-HCV positive EIA test result, either at admission or during their hemodialysis treatment period. Serum was collected and tested from 35 of these patients, of whom 26 had sufficient virus for sequencing and subtyping of NS5b region: eight of the nine patients with incident infection, 12 patients with chronic infection upon admission, and six patients whose HCV admission status was unknown. An HCV source patient was defined as an HCV-positive patient 1) with a >95% sequence identity match in the NS5b region of the HCV genome with a patient with incident infection and 2) who had received hemodialysis treatment on recurring days at the same time as the patient with incident infection, during the seroconverting patient's exposure period (i.e., from 6 months before the patient's last negative anti-HCV test through 2 weeks before the first positive anti-HCV test). The joint phylogenetic-epidemiologic analysis identified four different patients as the sources of HCV infection in four patients who seroconverted during 2005--2008 (all sequence identity matches between source and incident patients were >98%). Of the four source patients, one was among the nine with incident infection, two were among those with chronic HCV infection at admission, and one had unknown HCV infection status at admission. All four patients with incident infection and their respective source patients had dozens of treatment days in common (range: 59--121 days). Two of the four patients with incident infection had at least one treatment on the same dialysis machines as their HCV source patients; however, no record existed of the other two with incident infection having been treated during their incubation periods on the same machines as their source patients. HCV source patients could not be determined for five of the patients with incident infection because no sequence identity match was identified. None of the five had known HCV risk factors (e.g., occupational exposure, injection-drug use, high-risk sexual behaviors, or exposure to known HCV-positive persons). Two of the five reported no health-care exposures outside of the hemodialysis unit during their exposure periods; the other three reported respectively 1) one emergency department visit, 2) one hospitalization, and 3) one emergency department visit and two hospital admissions. Epidemiologic analysis is continuing in an effort to define narrower exposure periods and determine the mechanism or mechanisms of HCV transmission at this facility. Site InvestigationDuring the site investigation, NYSDOH documented inadequate HCV infection surveillance and patient follow-up (2). Numerous deficiencies in standard infection control practices also were identified (2). The hemodialysis unit did not obtain confirmatory testing for anti-HCV positive results, inform patients of their change in HCV infection status, report HCV seroconversions to the local health department, or provide patients with medical evaluation related to HCV infection. Contrary to CDC recommendations (2), monthly alanine aminotransferase (ALT) levels were not obtained from >90% of HCV-susceptible patients, and anti-HCV testing, although conducted on most patients, was performed at intervals ranging from once per month to once per 2 years rather than semiannually. Inadequate cleaning and disinfection practices were observed during site visits in July and August 2008. A single bleach-soaked gauze pad was used to clean a patient's entire dialysis station, including dialysis machine surfaces and ancillary patient equipment (e.g., blood pressure cuff and shared computer monitor and keyboard). The bleach solution was prepared and stored improperly, and staff members did not allow sufficient contact time between surfaces and bleach. Visible blood remained on dialysis chairs, dialysis machine surfaces, and the surrounding floor between patient treatments. Moreover, direct care staff members failed to don gloves with every patient encounter, change gloves between patients, or perform hand hygiene after contact with patients and soiled surfaces. Supervisory staff members failed to address these breaches. Many of the direct care staff members were unaware of the hemodialysis unit's written infection control policies, including those pertaining to cleaning and disinfection. Investigators also noted the lack of a separate clean area for medication storage and preparation and short turnover periods between patient treatments. On August 14, 2008, after evidence of ongoing infection control deficiencies and despite efforts at remediation, NYSDOH directed the hemodialysis unit to transfer all patients immediately to other facilities; all patients were transferred the next day. The hemodialysis unit subsequently surrendered its operating certificate and paid a $300,000 civil penalty to the state; the unit has not reopened. Based on evidence of HCV transmission since 2005, all patients who had received one or more treatments at the hemodialysis unit since January 23, 2004 (the date of the last facility survey in which no infection control deficiencies were observed) were notified by mail of the investigation and advised to be tested for HCV and other bloodborne pathogens (i.e., hepatitis B virus and human immunodeficiency virus). Notification letters were mailed on September 15, 2008, to a total of 657 patients from 37 states and two territories. As of January 11, 2009, no additional HCV seroconversions had been reported from health departments in New York, 13 other states, and one territory, accounting for 90% of the patients who were notified. Reported by: R Hallack, G Johnson, MS, E Clement, MSN, M Parker, PhD, J Schaffzin, MD, PhD, B Wallace, MD, P Smith, MD, New York State Dept of Health; ND Thompson, PhD, Div of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; PR Patel, MD, JF Perz, DrPH, Div of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases; J Magri, MD, Career Development Div, Office of Workforce and Career Development; JL Jaeger, MD, EIS Officer, CDC. Editorial Note:An estimated 3.2 million persons have chronic HCV infection, the most common chronic bloodborne infection in the United States (3). The prevalence of anti-HCV is estimated at 8% among chronic hemodialysis patients (4), compared with 1.6% in the U.S. population overall (3). HCV infection increases the risk for death among patients receiving chronic hemodialysis treatment and those undergoing renal transplantation (5). Many persons infected with HCV remain asymptomatic, although progression of underlying liver disease occurs in approximately 80% (6). Chronic HCV infection is the leading indication for liver transplantation in the United States (7). CDC recommendations for preventing HCV transmission in hemodialysis units were published in 2001 (Box) (2). Despite these recommendations, several hemodialysis-related HCV outbreaks have occurred in recent years; all involved breaches in infection control, and most were identified as a result of routine HCV screening (8). CDC recommends initial anti-HCV screening upon admission to the unit for all chronic hemodialysis patients. For HCV-susceptible patients, monthly ALT should be performed; anti-HCV screening should be obtained semiannually thereafter and in response to unexplained elevations in ALT, to facilitate early detection of transmission and implementation of control measures (2). Routine HCV screening of hemodialysis patients also is recommended by the National Kidney Foundation (9). However, dialysis providers are not reimbursed by Medicare for anti-HCV screening, and screening is not required by the Centers for Medicare and Medicaid Services (10). In the 2008 Medicare conditions for coverage for end stage renal disease facilities (10), CDC recommendations for preventing transmission of infections in hemodialysis units (2) were incorporated by reference, with the exception of screening for hepatitis C. The referenced recommendations have the authority of regulation. This investigation documented four cases of patient-to-patient transmission of HCV infection and identified five additional patients who might have acquired HCV infection while receiving treatment at the hemodialysis unit. Multiple possible mechanisms of HCV transmission were identified, including contaminated health-care worker hands and treatment surfaces. Contact transmission in the setting of extensive environmental contamination is a common mechanism for transmission of bloodborne pathogens in hemodialysis units (2). Because this investigation was restricted to patients undergoing treatment as of July 31, 2008, the actual number of incident cases at the hemodialysis unit might have been larger. This outbreak highlights the need for hemodialysis units to adhere to recommendations for infection control and comprehensive HCV surveillance, including routine anti-HCV screening, confirmatory testing of anti-HCV seroconversions, assessment of the adequacy of infection control practices in the setting of documented HCV seroconversion, and prompt reporting to the local health department as required by reportable disease laws or regulations. Had the hemodialysis unit in this report complied with these practices, HCV transmission might have been identified earlier, and control measures (e.g., reviewing infection control practices to identify potential mechanisms of transmission, ensuring adherence to unit infection control policies, and retraining direct care staff members) could have been implemented to interrupt further HCV transmission. Because many patients with HCV infection are asymptomatic, routine screening is essential to detect transmission within hemodialysis facilities and ensure that appropriate precautions are being followed consistently. Acknowledgments This report is based, in part, on contributions by E Rocchio, MA, K Southwick, MD, N Sureshbabu, and T Kwechin, New York State Dept of Health; and K Bornschlegel, MPH, New York City Dept of Health and Mental Hygiene. References
Figure 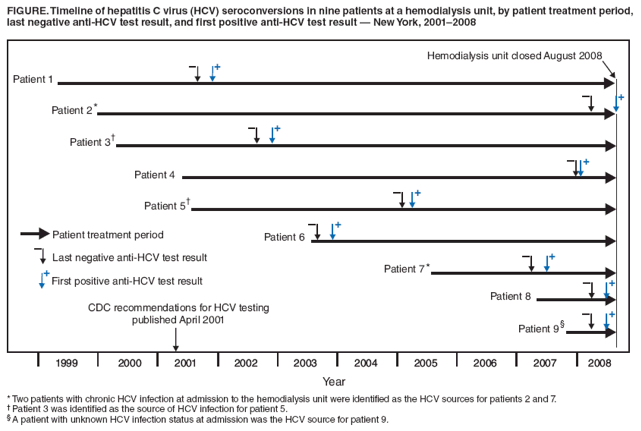 Return to top. Box  Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 3/5/2009 |
|||||||||
|