 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Potential Effects of Electronic Laboratory Reporting on Improving Timeliness of Infectious Disease Notification --- Florida, 2002--2006Electronic laboratory reporting (ELR) potentially can improve the timeliness of notifiable disease case reporting and subsequent disease control activities (1,2), but the extent of this improvement and the resulting effects on the workload of state or local surveillance teams are unknown. To estimate those effects, investigators from the Florida Department of Health (FDOH) evaluated the timeliness of reporting for four notifiable diseases of varying incubation periods: salmonellosis, shigellosis, meningococcal disease, and hepatitis A. Investigators then calculated the potential improvement expected with ELR using the assumption that ELR can reduce to 1 day the time from completion of a diagnostic laboratory test to notification of the county health department (CHD) of the result. This report summarizes the results of that analysis, which showed that ELR would reduce the total time from symptom onset to CHD notification of a case by nearly half for salmonellosis (from 12 days to 7 days) and shigellosis (from 10 days to 6 days), but would produce no change for meningococcal disease (4 days) and minimal improvement for hepatitis A (from 13 days to 10 days). In Florida, the benefits of ELR for reporting timeliness likely will vary by disease. The FDOH web-based reportable disease surveillance database (Merlin) was used to conduct this evaluation. The Florida Bureau of Epidemiology annually receives from CHDs approximately 30,000 reports of cases of notifiable infectious diseases. With full implementation of ELR, participating laboratories will transmit electronically all reportable laboratory results directly to Merlin, which can then be accessed by all CHD epidemiologists. To evaluate the potential of ELR to change the timeliness of disease reporting in Florida, FDOH investigators selected four key notifiable diseases, either because of high severity or significant public health concern: salmonellosis, shigellosis, meningococcal disease, and hepatitis A. Confirmed cases of the four diseases were analyzed for the study period, 2002--2006 (Box). Florida began implementation of ELR from commercial laboratories in 2006, but ELR was not in use for any of the four diseases included in this analysis during the study period. The regular practice during the study period was for CHD epidemiologists to manually enter all data for the four diseases into Merlin, including dates of symptom onset (as reported by patients), laboratory reporting, and CHD notification. Symptom onset date was defined as the date of illness reported by patient. Laboratory reporting date was the date the laboratory report was mailed, faxed, or conveyed to the CHD by telephone. CHD notification date was the date the CHD recorded receipt of the laboratory report. However, all three date fields were not required for a final case report to be submitted through Merlin to FDOH. Three time intervals were calculated: symptom onset date to laboratory reporting date (interval A), laboratory reporting date to CHD notification date (interval B), and symptom onset date to CHD notification date (interval C). Next, the percentage of cases reported within one and two incubation periods for each disease was calculated. Incubation period was used as a proxy for period of transmissibility (3). The incubation period for each of the four diseases (1 day for salmonellosis, 3 days for shigellosis, 4 days for meningococcal disease, and 30 days for hepatitis A) was assumed to be the midpoint of the range most commonly reported in the literature (4,5). Although the electronic transmission of laboratory reports is instantaneous, interval B was assumed to be 1 day when ELR was implemented because many laboratories batch their electronic reporting once per shift or once per day. Among the 23,263 confirmed cases of salmonellosis reported during the 5-year period, 81% of reports included symptom onset date, 72% included laboratory reporting date, and 96% included CHD notification date. Time intervals A, B, and C could be calculated for 57%, 68%, and 78% of cases, respectively (Table 1). The median number of days for intervals A, B, and C were 6, 5, and 12, respectively (Table 2). If ELR were used for salmonellosis reporting, interval B would decrease from 5 days to 1 day, which would result in a decrease of interval C (symptom onset date to CHD notification date) from 12 to 7 days (Table 2, Figure). The percentage reported within two incubation periods would increase from 1% to 10%. Reporting within two incubation periods would improve from 28% to 60% for shigellosis, from 84% to 94% for meningococcal disease, and from 92% to 98% for hepatitis A (Table 2). Reported by: A Kite-Powell, MS, JJ Hamilton, MPH, RS Hopkins, MD, Florida Dept of Health. JM DePasquale, MD, EIS Officer, CDC. Editorial Note:Investments in ELR systems for notifiable diseases generally are justified by expected improvements in timeliness and sensitivity of reporting. This analysis determined that, in Florida, improvements in timeliness in reporting would be highly disease specific. The greatest improvements in timeliness of reporting (i.e., the absolute number of days for CHDs to be notified of illness) would be expected for salmonellosis and shigellosis. In Florida, these two diseases often are reported by postal mail. With implementation of ELR, the reporting time from symptom onset to CHD notification would be nearly halved. These two diseases also would experience the greatest increase in timeliness of reporting within two incubation periods, in part because they have the shortest incubation periods of the four diseases tested in this analysis (1 and 3 days, respectively). When laboratory reports for a condition with a short incubation period are mailed from laboratory to CHD, only a small fraction of cases are reported to CHDs within two incubation periods. Results for meningococcal disease and hepatitis A, which have longer incubation periods, often are reported from laboratories by faster methods (fax or telephone). Because clinicians, laboratorians, and health departments already place great importance on timely reporting of meningococcal disease, more cases are reported within two incubation periods and the potential improvement with use of ELR is less. Under the assumptions of this analysis, the median number of days to report laboratory results to a CHD for meningococcal disease (1 day) would not change. In addition to timeliness, the completeness of reporting dates will improve for all conditions reported with ELR, approaching 100% for the laboratory reporting and CHD notification dates, which will be required fields automatically populated by ELR software. This information will permit time intervals to be more completely calculated. The date of symptom onset and any time interval using this date are, however, dependent on patient recall and not on programming of surveillance system software. The findings in this report are subject to at least three limitations. First, depending on the disease and category of date, from 1% to 33% of key dates were missing from the Merlin database (Table 1). Results might have differed if all such dates had been available for all cases. Second, interpretation and application of date field definitions by those entering data into Merlin vary, and this might lead to inconsistent data entry practices. Finally, Merlin does not allow for recording times in less than 1-day increments; thus, shorter intervals for reporting laboratory results could not be assessed. This analysis did not assess directly the potential effect of ELR on the workload of CHD communicable disease investigators in Florida. However, the implementation of ELR likely will have some effects. For example, most cases of meningococcal disease in Florida are reported quickly to FDOH and intensively investigated under the existing reporting system. Thus, the number of cases of meningococcal disease requiring investigation is not likely to increase with ELR. However, for salmonellosis, shigellosis, and hepatitis A, an increase in reported cases is anticipated with ELR, because all positive cultures and immunoglobulin (IgM) results will be transmitted electronically. Under the existing system, in 2005, 84% of hospitalized salmonellosis patients were recorded in the Merlin database, 73% of hospitalized shigellosis patients, and 52% of hospitalized hepatitis A patients. If ELR captures more cases than currently are entered into Merlin manually, the workload of county and state health staff will increase. For some reportable conditions (e.g., hepatitis A), a large amount of clinical information and additional laboratory data often is required to confirm the diagnosis after a laboratory report is received. Much of this information is not included among the ELR data but is needed to classify the case according to the case definition. The implementation of ELR might cause an increase in the number of preliminary reports that must be investigated, thus increasing workload, without a corresponding increase in the number of confirmed cases (6,7). ELR is being introduced in Florida in a stepwise fashion, with laboratory facilities brought on incrementally rather than all at once. This is allowing CHDs to assess the effects of ELR on workflow and human resource requirements for various reportable diseases. The analysis in this report suggests the effects of ELR will be disease specific, with differing limitations and challenges for each condition. Under a newly implemented ELR system, local and state public health officials should be able to 1) monitor timeliness and completeness of reporting, 2) assess workload and workflow, 3) ensure that reporting of high-priority conditions is not adversely affected by ELR, and 4) interact with clinicians in a manner that fosters respect for the clinician-patient relationship and compliance with state-mandated reporting requirements. If the number of reported cases increases substantially when ELR is implemented, jurisdictions will need to establish priorities for investigation and follow-up of laboratory reports received (6). References
Figure 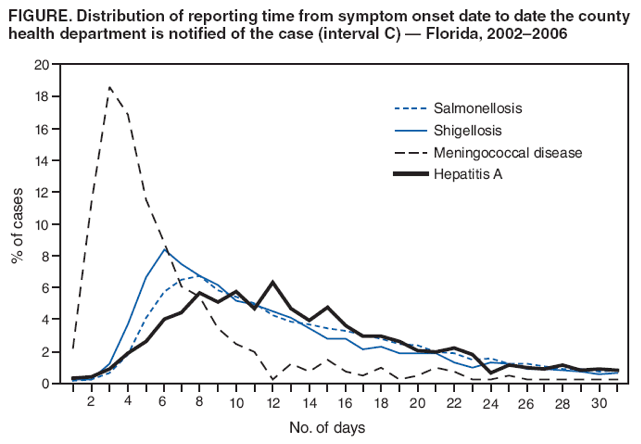 Return to top. Table 1 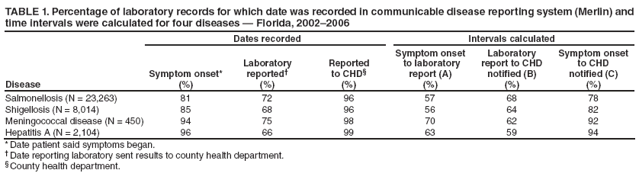 Return to top. Table 2  Return to top. Box 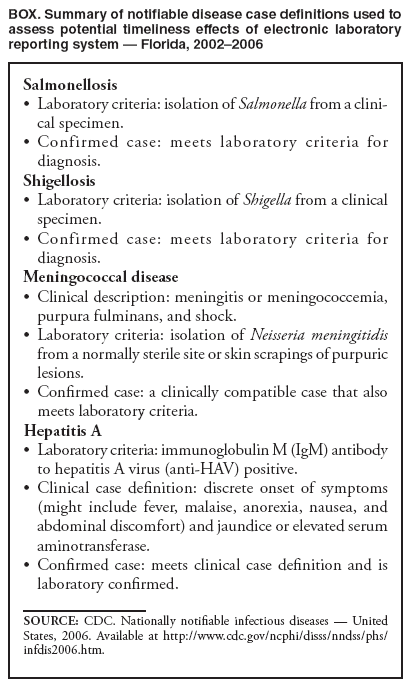 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/10/2008 |
|||||||||
|