 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. West Nile Virus Activity --- United States, 2007West Nile virus (WNV) is the leading cause of arboviral encephalitis in the United States. Originally identified in Africa in 1937, WNV was first detected in the western hemisphere in 1999 in New York City. Since then, WNV has caused seasonal epidemics of febrile illness and neurologic disease in the United States. This report summarizes national WNV surveillance data for 2007. WNV transmission to humans or animals expanded into 19 counties that had not reported transmission previously and recurred in 1,148 counties where transmission had been reported in previous years. A total of 1,227 cases of WNV neuroinvasive disease (WNND) and 117 deaths were reported. These findings highlight the need for ongoing surveillance, mosquito control, promotion of personal protection from mosquito bites, and research into additional prevention strategies, including a WNV human vaccine. WNV data are reported to CDC through ArboNET, an Internet-based arbovirus surveillance system managed by state health departments and CDC. State and local health departments 1) collect reports from health-care providers and clinical laboratories regarding cases of WNV disease in humans; 2) collect reports of WNV presumptive viremic blood donors (PVDs)* from blood collection agencies; 3) collect and test dead birds, often focusing on corvids (e.g., crows, jays, and magpies), which have high mortality attributed to WNV infection; 4) collaborate with veterinarians to collect reports of WNV infection in nonhuman mammals; and 5) collect mosquitoes to test for evidence of WNV infection. Human WNV disease cases are classified as 1) WNND (i.e., meningitis, encephalitis, or acute flaccid paralysis); 2) West Nile fever (WNF), which is symptomatic WNV disease that does not affect the nervous system; or 3) an unspecified clinical syndrome. WNF reporting is highly variable by jurisdiction, depending on the level of interest in reporting and use of diagnostic testing; therefore, most of this report focuses on WNND cases, which are thought to be more consistently identified and reported because of the severity of the illness. Human SurveillanceDuring 2007, a total of 3,630 cases of WNV disease in humans were reported from 775 counties in 44 states (i.e., 25% of the 3,142 counties in the United States). Of these cases, 1,227 (34%) were WNND, 2,350 (65%) were WNF, and 53 (1%) were unspecified clinical syndromes. A total of 352 PVDs were identified through routine screening of the blood supply. Of these PVDs, 281 (80%) were asymptomatic, five (1%) subsequently developed WNND, and 66 (19%) subsequently had WNF. Overall, the incidence of WNND in the United States was 0.4 per 100,000 population. The highest incidence of WNND occurred primarily in the west-central United States (Figure 1); the five states with highest incidence were North Dakota (7.7 cases per 100,000 residents), South Dakota (6.2), Wyoming (4.6), Montana (4.0), and Colorado (2.2). Among all states, WNND peaked during the first week in August, and 1,086 (89%) cases were reported during July--September (Figure 2). This seasonality was consistent with trends observed in the preceding 7 years. Of the 1,227 WNND cases, 729 (59%) occurred in males. The median age of patients was 57 years (range: 1 month--97 years), with increasing incidence among older age groups (Figure 3). Overall, 1,089 (89%) patients were hospitalized (median age: 59 years; range: 1 month--97 years), and 117 (10%) died (median age: 77 years; range: 43--96 years). A total of 765 (62%) WNND cases were classified as encephalitis, 452 (37%) as meningitis, and 63 (5%) as acute flaccid paralysis; 53 of these cases were classified as acute flaccid paralysis coincident with encephalitis or meningitis. Animal SurveillanceIn 2007, a total of 2,182 dead WNV-infected birds were reported from 315 counties in 35 states and Puerto Rico; 157 counties in 28 states and Puerto Rico reported infected birds but no clinically apparent human disease. The number of reported WNV-infected birds peaked during the first week of September. Corvids accounted for 1,690 (77%) of the birds; most states targeted corvids for surveillance. Since 1999, WNV infection has been reported in 321 avian species, including four species (Bronzed Cowbird, Cackling Goose, Le Conte's Thrasher, and Northern Pintail) in which WNV was identified for the first time during 2007. Of 507 reported cases of WNV disease among nonhuman mammals, 471 (93%) occurred in equines, and 36 (7%) occurred in other species (squirrels [27], canines [five], and unspecified species [four]). Equine cases were reported from 320 counties in 35 states and Puerto Rico; Texas reported 20% of all equine cases. The number of reported WNV-infected equines peaked in mid-August. Mosquito SurveillanceA total of 8,215 mosquito pools from 371 counties in 39 states, the District of Columbia, and Puerto Rico tested positive for WNV. Among the WNV-positive pools, 6,286 (77%) were made up of Culex mosquitoes thought to be the principal vectors of WNV transmission (e.g., Cx. pipiens, Cx. quinquefasciatus, Cx. restuans, Cx. salinarius, and Cx. tarsalis). Unidentified or other species of Culex mosquitoes made up 1,746 (21%) pools, and non-Culex species (e.g., Aedes spp., Anopheles spp., Coquillettidia perturbans, Culiseta spp., and Uranotaenia sapphirina) made up 106 (1%) pools. Data from 2007 included the first report of WNV infection in Culex bahamensis, which was collected in Puerto Rico. The number of reported WNV-infected mosquito pools peaked during mid-August. Reported by: NP Lindsey, MS, JA Lehman, JE Staples, MD, N Komar, ScD, E Zielinski-Gutierrez, DrPh, EB Hayes, MD, RS Nasci, PhD, M Fischer, MD, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; M Duffy, DVM, EIS Officer, CDC. Editorial Note:In 2007, the reported incidence of WNND in the United States was 0.4 per 100,000 population. This incidence is similar to that reported in 2004 (0.4), 2005 (0.4), and 2006 (0.5), but substantially lower than the reported incidence for 2002 (1.0) and 2003 (1.0) (1,2). The relative stability in the number of reported WNND cases during the past 4 years likely represents endemic WNV transmission in the continental United States. However, because of variation in vectors, avian amplifying hosts, human activity, and environmental factors (e.g., temperature and rainfall), predicting future WNV transmission intensity is difficult (3,4). Reported cases of WNND are thought to be the most accurate indicator of WNV activity in humans. WNND reporting is thought to be more complete because of substantial associated morbidity and mortality, whereas WNF likely is underdiagnosed and underreported. Serologic surveys indicate that approximately 20% of WNV infections result in WNF and 0.7% of WNV infections result in WNND (5). Based on these estimates, approximately 175,000 WNV infections and 35,000 WNF cases occurred in the United States in 2007. Only 2,350 WNF cases were reported to ArboNET in 2007, representing <10% of the estimated number of WNF cases. In 2007, evidence of WNV human disease again was detected in all geographic regions of the continental United States. Although the highest incidence of WNND continued to occur in the west-central United States (6), Idaho reported only 10 WNND cases in 2007, a 93% decrease from the 139 cases reported in 2006 (7). This illustrates the wide annual variability and focality of WNV transmission. Human WNV infection was identified for the first time in Puerto Rico in 2007 among three asymptomatic blood donors (8). ArboNET integrates arboviral diagnostic testing and reporting to produce timely, actionable data that public health professionals use to tailor effective prevention and control messages at the local level. Continued surveillance is important in monitoring potential changes in WNV epidemiology and for providing early warning for local WNND outbreaks. In addition, ArboNET is well positioned to help identify and manage future introductions of exotic arboviruses. For example, cases of ill travelers entering the United States who are likely viremic with nonendemic arboviruses (e.g., dengue virus and chikungunya virus) are reported to ArboNET (9). WNV vaccines are licensed for use in horses and are being evaluated currently in phase 2 human clinical trials (10). Because no WNV vaccine is available currently for use in humans, prevention depends on personal protective measures. Use of repellents containing DEET, picaridin, oil of lemon eucalyptus, or IR3535 provides effective protection against mosquitoes. Long-sleeved shirts, long pants, and socks provide barrier protection against mosquito bites, and many fabrics can be treated with permethrin to provide an additional level of protection. Avoiding outdoor exposure during dusk and dawn, when Culex mosquito species are more active, will decrease the likelihood of WNV exposure. Household measures, such as installing and repairing window screens and covering or draining water-holding containers to reduce mosquito breeding sites, can decrease further the risk for WNV exposure. Additional information on effective prevention of WNV infection is available from CDC at http://www.cdc.gov/ncidod/dvbid/westnile/index.htm. An overview of current year WNV transmission activity is available at http://diseasemaps.usgs.gov/wnv_us_human.html. Acknowledgments This report is based, in part, on data provided by ArboNET surveillance coordinators in local and state health departments and ArboNET technical staff, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, CDC. References
* A PVD is a person whose blood tested positive when screened for the presence of WNV. PVDs are followed up by the blood collection agency with additional tests to verify their infection. Some PVDs go on to develop symptoms after donation, at which point they are considered to have WNV disease. A sample of mosquitoes (usually no more than 50) of the same species and sex, collected within a defined sampling area and period. Figure 1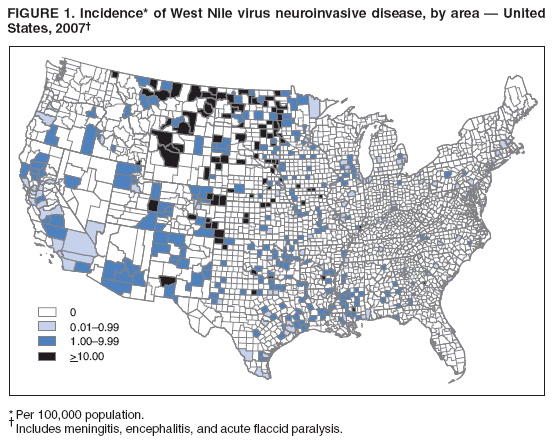 Return to top. Figure 2 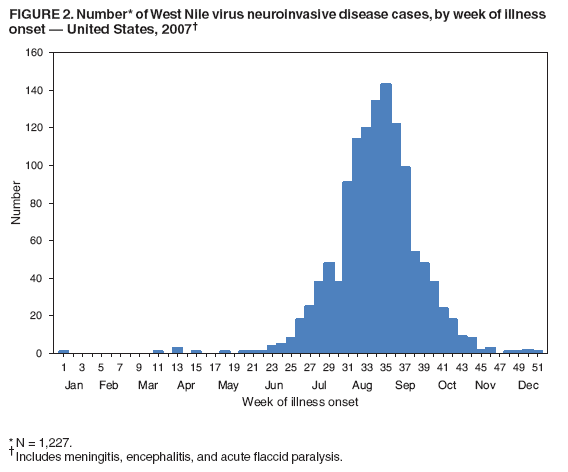 Return to top. Figure 3 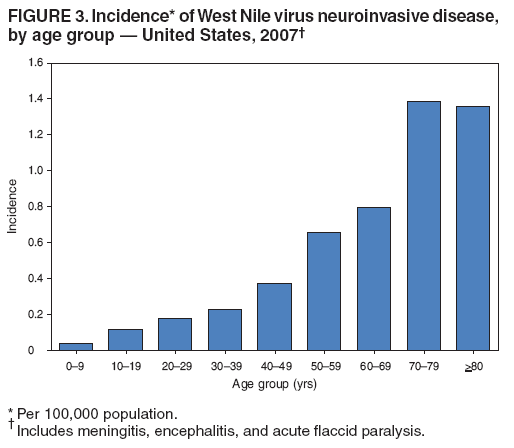 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 7/3/2008 |
|||||||||
|