 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Multistate Outbreak of Mumps --- United States, January 1--May 2, 2006CDC and state and local health departments continue to investigate an outbreak of mumps that began in Iowa in December 2005 (1) and involved at least 10 additional states as of May 2, 2006. This report summarizes preliminary data reported to CDC from these 11 states and provides recommendations to prevent and control mumps during an outbreak. Cases of mumps are reportable through the National Notifiable Diseases Surveillance System (NNDSS) (2). NNDSS reports are transmitted electronically to CDC each week and include information on individual cases such as age, sex, date of symptom onset, vaccination status, and complications of illness. Mumps cases included in this report are those with onset from January 1 (MMWR week 1) through April 29 (MMWR week 17) that were reported to CDC as of May 2 through NNDSS (or the Iowa mumps outbreak-specific reporting system) from Iowa and 10 additional states that reported one or more cases of mumps epidemiologically linked to the multistate outbreak. In addition to cases reported through NNDSS, to provide information rapidly during this outbreak, states have been reporting aggregate numbers of mumps cases and mumps-related hospitalizations and complications biweekly to CDC. Cases reported in this manner through May 2, 2006, also are included in this report. The clinical case definition of mumps* is an illness with acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary gland, lasting 2 or more days, and without other apparent cause. A confirmed case of mumps is one that is laboratory confirmed or meets the clinical case definition and is linked epidemiologically to a confirmed or probable case. A case is classified as probable if it meets the clinical case definition but is neither laboratory-confirmed nor linked to another confirmed or probable mumps case. In accordance with these definitions, asymptomatic, laboratory confirmed infections were counted as confirmed cases in all states except Iowa. In Iowa, laboratory-confirmed cases that were asymptomatic or had clinical information pending, and cases for which high suspicion for mumps existed but case classification was not yet determined were classified as suspect. During January 1--May 2, 11 states reported 2,597 cases of mumps. Eight states (Illinois, Iowa, Kansas, Missouri, Nebraska, Pennsylvania, South Dakota, and Wisconsin) reported mumps outbreaks with ongoing local transmission or clusters of cases; three states (Colorado, Minnesota, and Mississippi) reported cases associated with travel from an outbreak state. The majority of mumps cases (1,487 [57%]) were reported from Iowa; states with the next highest case totals were Kansas (371), Illinois (224), Nebraska (201), and Wisconsin (176) (Figure 1). Of the 2,597 cases reported overall, 1,275 (49%) were classified as confirmed, 915 (35%) as probable, and 287 (11%) as suspect; for 120 (5%) cases, classification was unknown. Twelve mumps viral isolates from six states were characterized; all were mumps genotype G. For 2,067 (80%) of the 2,597 mumps cases with patient age available, the median age was 21 years (range: <1 year to 96 years). In the eight states with outbreaks, the incidence rate was highest among persons aged 18--24 years (17.1 per 100,000 population), followed by persons aged 5--17 years (5.2) and 25--39 years (4.8) (Figure 2). Among the 2,073 patients for whom sex was known, 1,244 (60%) were female. Among the 2,073 cases for which week of onset was known, 1,426 (69%) were reported in April (Figure 3). The peak week of onset has been April 2--8 (week 14) in Iowa and April 16--22 (week 16) in other states. However, additional cases with onset dates in April continue to be reported. Parotitis was reported in 870 (66%) of the 1,327 patients for whom such data were available. Data regarding mumps complications and hospitalizations are incomplete. However, complications have included 27 reports of orchitis, 11 meningitis, four encephalitis, four deafness, and one each of oophoritis, mastitis, pancreatitis, and unspecified complications. A total of 25 hospitalizations were reported, but insufficient data were provided to determine whether mumps caused all the hospitalizations. No deaths have been reported. Vaccination status of reported mumps patients is being ascertained. In Iowa, preliminary vaccination data were reported through May 3, 2006. Among 1,192 patients, 69 (6%) were unvaccinated, 141 (12%) had received 1 dose of measles, mumps, and rubella (MMR) vaccine, and 607 (51%) had received 2 doses of MMR vaccine; the vaccination status of 375 (31%) patients, the majority of whom were adults who did not have vaccination records, was unknown. Preliminary data, as of April 10, from two mumps outbreaks on college campuses in an Iowa county affected early in the outbreak, identified attack rates of reported mumps cases§ of 2.0% (31 of 1,542 students) and 3.8% (44 of 1,168 students). Preliminary data from vaccine coverage surveys suggest that the college with the higher attack rate had a smaller proportion (77% versus 97%) of students documented as having received 2 doses of MMR vaccine. As of May 10, a total of 11 persons potentially infected with mumps who traveled by aircraft during March 26--April 25 had been identified on 33 commercial flights operated by eight different airlines. Notifications had either been initiated or completed for persons potentially exposed on all identified flights. As of May 12, of approximately 575 persons potentially exposed on the flights, 132 had received follow-up >25 days after their potential exposure. Two cases of mumps were identified, possibly associated with transmission during air travel. Both cases occurred among Iowa residents, one of whom was a traveling companion of a person known to have mumps. Reported by: K Gershman, MD, S Rios, D Woods-Stout, Colorado Dept of Public Health and Environment. M Dworkin, MD, K Hunt, Illinois Dept of Public Health. DC Hunt, MPH, J Hill, MPH, Kansas Dept of Health and Environment. P Quinlisk, MD, M Harris, MPH, Iowa Dept of Public Health. C Kenyon, MPH, Minnesota Dept of Health. C Evans, K Mills McNeill, MD, PhD, RG Travnicek, MD, Mississippi Dept of Health. B Zhu, MD, E Hedrick, HL Marx Jr, R Renicker, MSA, Missouri Dept of Health and Senior Svcs. AL O'Keefe, MD, T Safranek, MD, Nebraska Health and Human Svcs System. S Slagy, S Silvestri, Allegheny County Health Dept; J Sullivan, York City Health Bur; J Mankowski, Erie County Health Dept; R Grill, K Luckenbill, P Lurie, MD, R Rickert, MPH, H Stafford, Pennsylvania Dept of Health. S Gannon, L Kightlinger, PhD, South Dakota Dept of Health. J Berg, J Davis, MD, J Gabor, Wisconsin Dept of Health and Family Svcs. F Averhoff, MD, K Marienau, MD, Div of Global Migration and Quarantine; M Bell, MD, E Bolyard, MPH, C McDonald, MD, A Srinivasan, MD, Div of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases (proposed); TA Santibanez, PhD, and J Santoli, MD, Immunization Svcs Div, SW Roush, MPH, PU Srivastava, MS, Div of Bacterial Diseases, L Anderson, MD, B Bellini, PhD, CB Bridges, MD, G Dayan, MD, ST Goldstein, MD, M Marin, MD, U Parashar, MD, S Redd, S Reef, MD, J Rota, MPH, PA Rota, PhD, J Seward, MBBS, C Shawney, Div of Viral Diseases, National Center for Immunization and Respiratory Diseases (proposed); A Huang, MD, A Parker, MSN, MPH, T Shimabukuro, MD, EIS officers, CDC. Editorial Note:In the United States, the reported incidence of mumps declined after introduction of mumps vaccine in 1967 and the recommendation for its routine use in 1977 (3). After expanded recommendations for a 2-dose MMR vaccine schedule for measles control in 1989 (3), mumps cases declined further (Figure 4). During 2001--2003, fewer than 300 mumps cases were reported each year, a 99% decline from the 185,691 cases reported in 1968 (2). The current multistate mumps outbreak, with 2,597 cases reported through May 2, 2006, is the largest number of mumps cases reported to CDC in a single year since 1991, when 4,264 cases were reported (2). The first cases in the current outbreak were detected on a college campus in eastern Iowa in December 2005; the source of these initial cases is unknown (1). Although the age group most affected (38% of cases) has been young adults aged 18--24 years, many of whom are college students, the outbreak has spread to all age groups (1). Multiple factors might have contributed to the spread of mumps in this outbreak and on college campuses. First, the college campus environment (e.g., living in dormitories with frequent and extended close contact with other students) facilitates transmission of mumps and other illnesses that are spread through respiratory and oral secretions. Second, only 25 states¶ and the District of Columbia report a college admission requirement of 2 doses of MMR vaccine, including three of the 11 states with outbreak-associated cases of mumps; no data on implementation and evaluation of the 2-dose college admission requirement are available (CDC, unpublished data, 2006). Thus, 2-dose coverage with mumps-containing vaccine among college students likely is lower than the median 97% (range: 57%--99%) coverage for measles-containing vaccine (almost exclusively administered as MMR vaccine) for students entering elementary school and the median 98% (range: 62%--99%) coverage for students entering middle school reported in 2000 from 38 and 25 states, respectively (4). Third, delayed recognition and diagnosis of mumps cases might have contributed to the spread in this outbreak; younger physicians in the United States likely have not seen mumps, and physicians might not consider the diagnosis in vaccinated persons. Fourth, 2 doses of MMR vaccine are not 100% effective in preventing disease, and accumulation of susceptible persons who were not successfully immunized might be sufficient to sustain transmission in certain settings. In addition, the vaccine might be less effective in preventing asymptomatic infection or atypical mumps than in preventing parotitis, and persons with asymptomatic infection or mild disease might contribute to transmission. Finally, waning immunity has been postulated as a contributing factor in this outbreak. Young adults aged 18--24 years would most commonly have received their most recent dose of mumps-containing vaccine (i.e., MMR vaccine) 6--17 years ago. High vaccination coverage with 2 doses of MMR vaccine, especially in school-aged populations in the United States, likely prevented thousands of additional cases of mumps in this outbreak. Postlicensure studies conducted in the United States during 1973--1989 determined that 1 dose of mumps or MMR vaccine was 75%--91% effective in preventing mumps with parotitis that lasts >2 days (5). Although fewer data are available on the effectiveness of 2 doses of MMR vaccine against mumps, one study from the United Kingdom documented vaccine effectiveness of 88% with 2 doses (6). In a mumps outbreak in a high school in Kansas, students vaccinated with 1 dose of MMR vaccine had an attack rate five times that of students vaccinated with 2 doses (7). In a mumps outbreak in a middle school in 1982, before mumps vaccination became widespread, attack rates of 25%--49% occurred among unvaccinated students, depending on how cases were ascertained (8). During 1986--1990, after widespread implementation of a 1-dose mumps vaccination policy, attack rates of 2%--18% (most >6%) were documented in mumps outbreaks among junior high and high school students with vaccination coverage of >95% (7,9). In contrast, preliminary data from two colleges in Iowa during the current outbreak identified attack rates of 2.0% and 3.8%, respectively, with the lower attack rate in the college with higher 2-dose vaccination coverage. To prevent mumps, the Advisory Committee on Immunization Practices (ACIP) recommends a 2-dose MMR vaccination series for all children, with the first dose administered at ages 12--15 months and the second dose at ages 4--6 years (3). Two doses of MMR vaccine are recommended for school and college entry unless the student has other evidence of immunity (3). In a specially convened meeting on May 17, 2006, ACIP redefined evidence of immunity to mumps through vaccination as follows: 1 dose of a live mumps virus vaccine** for preschool children and adults not at high risk; 2 doses for children in grades K--12 and adults at high risk (i.e., persons who work in health-care facilities, international travelers, and students at post-high school educational institutions). Other criteria for evidence of immunity (i.e., birth before 1957, documentation of physician-diagnosed mumps, or laboratory evidence of immunity) are unchanged. Furthermore, health-care facilities should consider recommending 1 dose of MMR vaccine to unvaccinated health-care workers born before 1957 who do not have other evidence of mumps immunity. During an outbreak and depending on the epidemiology of the outbreak (e.g., the age groups and/or institutions involved), a second dose of vaccine should be considered for adults and for children aged 1--4 years who have received 1 dose. The second dose should be administered as early as 28 days after the first dose, the minimum recommended interval between 2 MMR vaccine doses. In addition, during an outbreak, health-care facilities should strongly consider recommending 2 doses of MMR vaccine to unvaccinated workers born before 1957 who do not have other evidence of mumps immunity. An MMWR Notice to Readers will be published, summarizing these interim recommendations in more detail. Additional means to decrease transmission in outbreak settings include exclusion of persons without evidence of immunity to mumps from institutions such as schools and colleges that are affected by the outbreak. Once vaccinated, students and staff can be readmitted to school immediately, even if they have been exposed to a case of mumps. The period of exclusion for those who remain unvaccinated is 26 days after the onset of parotitis in the last person in the affected institution. Students who acquire mumps illness should be excluded from school until 9 days after the onset of parotitis. After an exposure to mumps, unvaccinated health-care workers without evidence of immunity should be vaccinated and excluded from duty from the 12th day after the first exposure through the 26th day after the last exposure. Health-care workers with mumps illness should be excluded from work until 9 days after the onset of parotitis. In response to the current outbreak, the Iowa Department of Public Health (IDPH) issued vaccination recommendations in March targeting college campus and health-care worker populations at high risk. On April 14, CDC issued a Health Advisory Notice summarizing vaccine policy recommendations for mumps prevention and control. In conjunction with local health departments, IDPH launched a statewide vaccination campaign during April 24--26, targeting persons aged 18--22 years in the 35 Iowa counties with the state's largest colleges and universities. In the second phase of the campaign, conducted May 2--4, vaccination was expanded to the remaining 64 counties, targeting persons aged 18--25 years. A third phase of the vaccination campaign was begun May 10 and targets persons aged 18--46 years. Vaccination activities also are being conducted or planned in Kansas, South Dakota, and Wisconsin. The data presented in this report are preliminary; the case count is likely to change as additional data become available. Certain reported cases might not have been caused by mumps; cases in persons without parotitis might have been misclassified on the basis of serologic tests. Because of the low number of reported mumps cases during the last decade, laboratorians have limited experience with mumps tests, particularly IgM antibody tests (10). Several different mumps IgM antibody tests are in use; however, neither the sensitivities nor specificities of these tests when used with serum specimens from either unvaccinated or vaccinated persons have been clearly defined. Consequently, interpretation of these antibody test results is difficult, especially in previously vaccinated persons. Studies to define the sensitivity and specificity of mumps IgM antibody tests and reverse transcription--polymerase chain reaction (RT-PCR) tests for mumps virus RNA are in progress. CDC continues to work with state and local health departments to conduct mumps surveillance, assist with prevention and control activities, and evaluate vaccine effectiveness, duration of immunity, and risk factors for mumps illness. References
* Available at http://www.cste.org/ps/1999/1999-id-09.htm. Available at http://www.idph.state.ia.us/adper/common/pdf/mumps/mumps_update_050406.pdf. § Defined as isolation of mumps virus from a clinical specimen; parotitis or orchitis; or submaxillary or submental swelling. ¶ Arizona, Arkansas, Colorado, Connecticut, Delaware, Georgia, Hawaii, Illinois, Indiana, Kansas, Louisiana, Massachusetts, Mississippi, Montana, Nevada, New York, North Carolina, North Dakota, Oklahoma, Oregon, Rhode Island, Tennessee, Texas, Vermont, and Virginia. ** Combined MMR vaccine generally should be used whenever any of its component vaccines are indicated. For children aged 1--12 years, MMRV vaccine can be considered if varicella vaccine is indicated. Figure 1 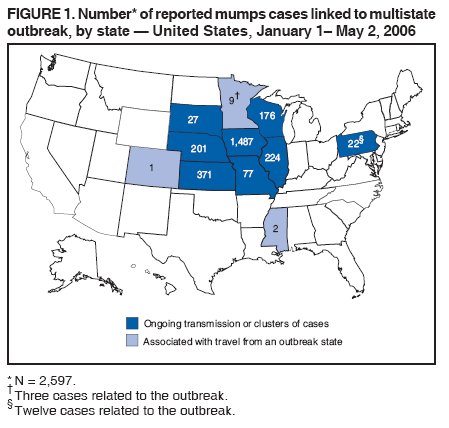 Return to top. Figure 2 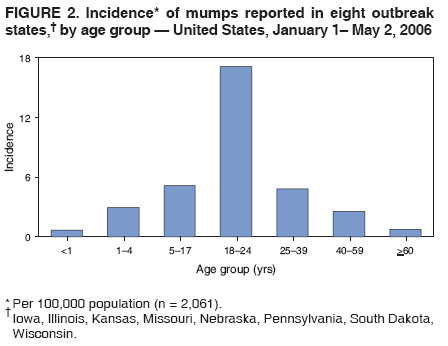 Return to top. Figure 3 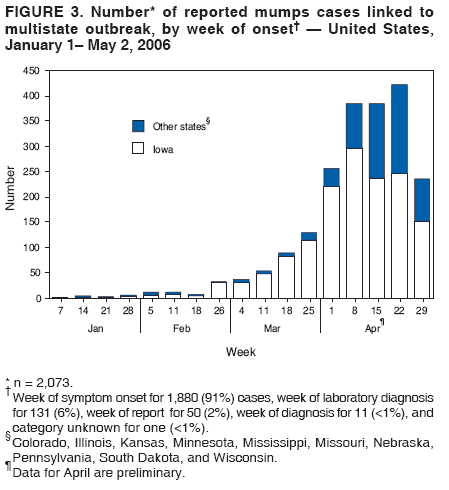 Return to top. Figure 4 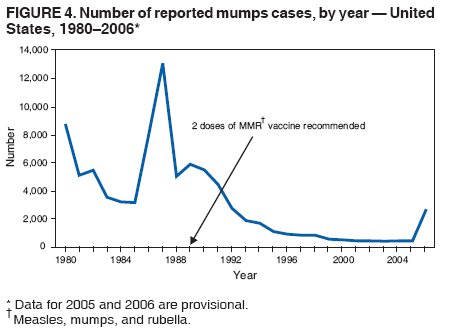 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/18/2006 |
|||||||||
|