 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Immunization Information Systems Progress --- United States, 2005Immunization registries are confidential, computerized information systems that collect and consolidate vaccination data from multiple health-care providers, generate reminder and recall notifications, and assess vaccination coverage within a defined geographic area (1,2). A registry with added capabilities, such as vaccine management, adverse event reporting, lifespan vaccination histories, and linkages with electronic data sources, is called an immunization information system (IIS) (3). This report summarizes data from CDC's 2005 Immunization Information System Annual Report (IISAR), a survey of grantees in 50 states, five cities, and the District of Columbia (DC) that receive funding under section 317b of the Public Health Service Act. These data indicated that approximately 56% of U.S. children aged <6 years participated in an IIS, an increase from 48% in 2004. Moreover, 75% percent of public vaccination provider sites and 44% of private vaccination provider sites submitted vaccination data to an IIS during July--December 2005. These findings underscore the need to increase the number of participating children, from the current 13 million to approximately 21 million, to assure 95% participation of children aged <6 years and improve the effectiveness of U.S. immunization programs. The 2005 IISAR, a self-administered, Internet-based questionnaire, was available to immunization program managers as part of an annual reporting requirement. As in previous years, respondents were asked about the number of children aged <6 years participating in the IIS, the number of health-care provider sites participating in the IIS, and other programmatic and technical capabilities (e.g., data linkages with other public health programs, data use, vaccine management, software and hardware capabilities, and reporting functions). All 56 grantees were asked to complete the questionnaire; 52 reported on the number of children aged <6 years participating in an IIS. Estimates of the total number of children aged <6 years were based on 2005 U.S. census data. The findings indicated that, of approximately 23 million U.S. children aged <6 years, an estimated 13 million (56%) participated in an IIS. Eleven (20%) IIS grantees (Alabama, Arkansas, Arizona, Delaware, Michigan, Mississippi, New York City, North Dakota, Oregon, Philadelphia, and Wisconsin) had >95% of children aged <6 years participating in an IIS (Figure). Eleven (20%) other IIS grantees (DC, Idaho, Iowa, Louisiana, Missouri, Montana, Oklahoma, Rhode Island, Tennessee, Utah, and Washington) had participation ranging from 81% to 94%. Approximately 75% of public vaccination provider sites and 44% of private vaccination provider sites submitted vaccination data to an IIS during July--December 2005.§ Twenty-two (39%) grantees reported that >95% of public vaccination provider sites submitted vaccination data to an IIS; eight (14%) reported submission of vaccination data by 81%--94% of public provider vaccination sites. Eight (14%) grantees (Arkansas, Connecticut, DC, Mississippi, North Dakota, Philadelphia, San Antonio, and South Dakota,) reported that >95% of private vaccination provider sites submitted vaccination data to an IIS; five (9%) (Arizona, Delaware, Michigan, Oregon, and Wisconsin) reported data submission by 81%--94% of private provider vaccination sites. Reported by: BC Canavan, M Kurilo, MPH, Oregon Dept of Human Svcs. T Moss, Georgia Registry Immunization Transactions and Svcs. R McLaren, MS, K Berry, MD, District of Columbia Dept of Health. C Thomas, Trey Industries, Locust Grove, Virginia. B Rasulnia, MPH, J Kelly, G Urquhart, MPH, Immunization Svcs Div, National Center for Immunization and Respiratory Diseases (proposed), CDC. Editorial Note:In 2005, approximately 56% of U.S. children aged <6 years participated in an IIS, an increase from 48% in 2004, or approximately 2 million more children (3). In addition, IIS private-provider--site participation increased from 39% in 2004 to 44% in 2005. IISs are being used increasingly as a decision-making tool for immunization programs and health-care providers to generate patient reminders and recalls, perform vaccine inventory management and distribution tasks, conduct routine public health surveillance, conduct school assessments, and identify clusters of undervaccinated children. Data from IISs have been used by immunization programs to make more effective and timely decisions. For example, during a routine Vaccines for Children Program site visit, the Oregon Immunization Program discovered that one vaccine (diphtheria and tetanus toxoids and acellular pertussis vaccine [DTaP]) was not being stored at proper temperatures in a pediatric clinic. According to data in the Oregon IIS, approximately 3,100 children had received 1 or more doses of DTaP or TriHIBit®¶ (Sanofi Pasteur, Swiftwater, Pennsylvania) vaccine during the period in which the vaccines were improperly stored. Within 8 days, Oregon IIS staff members had coordinated with the clinic to compile the necessary information to conduct a patient recall. An estimated 3,100 families received notices to return for revaccination; 1,280 (41%) children returned to the clinic and received 1 or more doses of vaccine containing diphtheria and tetanus within 90 days after the notice was mailed. The ability to share and exchange data with other information systems also has increased the usefulness of IISs for health insurance providers, health department clinics, Medicaid, and schools. The ability to use IIS data to comply with school-entry laws has ensured up-to-date vaccinations for children and improved the quality of IIS data. In 2005, a total of 38 (75%) grantees provided elementary schools with access to IIS data to monitor, document, and comply with school entry laws. In 2003, the Georgia Registry of Immunization Transactions and Services (GRITS) formed a partnership with the Houston Hot Shots Coalition in Houston County, Georgia, to increase use of GRITS in kindergarten classes and elementary schools in Houston County. Before 2003, annual kindergarten up-to-date vaccination rates for the Houston County Board of Education ranged from 67% to 90%. After implementing the partnership's recommendation to use GRITS for the 2003--04 school year audit, the rate for all 22 elementary schools was 100%. As a result of this success, the coalition presented the school superintendent with a proposal that GRITS be the official school-vaccination record for all students and that all students entering Houston County schools have their vaccination records validated by GRITS. The coalition proposal was approved by the school superintendent and implemented for the 2006--07 school year. In DC, the Department of Health collaborated with DC Public Schools (DCPS) and other partners on the DC School Immunization Project, which successfully monitored and documented school vaccination rates for the estimated 54,000 children enrolled in DCPS. The project objectives were to 1) use local partnerships to link traditional and high-technology quality-improvement strategies to overcome limited resources and achieve higher school vaccination rates; 2) identify and track vaccination levels for all public school children; and 3) use the IIS for quality improvement and improvement of overall vaccination rates and accuracy. DCPS provided the IIS with weekly enrollment files, which kept IIS staff members informed about new enrollees and allowed them to update vaccination rates. In addition, health insurance providers in the DC area provided the IIS with electronic enrollment data monthly, and the IIS provided updated information on the vaccination status of enrollees, including a list, by school, of enrolled students who met vaccination requirements. Before 2001, IISs were used for assessing DCPS vaccination compliance for entry to school and middle schools only. After implementing schoolwide policies to use IISs, the proportion of students with documentation of DCPS-required vaccinations increased from 40% in June 2001 to 96% in June 2006. At the end of the 2005--2006 school year, 155 (98%) of 158 schools in DCPS had compliance rates of >90%, and 28 had rates of 100%. The findings in this report are subject to at least two limitations. First, data from the 2005 IISAR are self-reported, which might have resulted in reporting bias. Second, because some grantees did not report data, the IIS participation rates for children aged <6 years and providers might be underestimated or overestimated. Immunization programs that use IIS data have improved the quality of vaccination activities in various settings in Oregon, Georgia, and DC. These examples illustrate the usefulness of IIS data for assessing program activities and measuring progress toward reaching immunization program goals. As participation in IIS increases and data quality improves, data from IIS will improve the effectiveness and efficiency of immunization programs throughout the United States. References
* Participation is defined as having two or more recorded vaccinations. Chicago, Illinois; Houston, Texas; New York City, New York; Philadelphia, Pennsylvania; and San Antonio, Texas. § Number of provider vaccination sites (public and private) is based on grantee self-reports. ¶ Combination Haemophilus influenzae B conjugate vaccine (ACTHib® [Sanofi Pasteur]) reconstituted with DTaP (Tripedia® [Sanofi Pasteur]). Figure 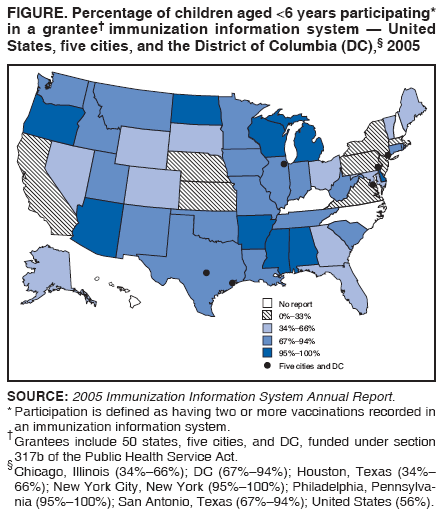 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/13/2006 |
|||||||||
|