 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Hydrogen Cyanamide--Related Illnesses --- Italy, 2002--2004Hydrogen cyanamide* is used in agriculture as a plant growth regulator and is applied to many deciduous plants to stimulate uniform budbreak after dormancy, resulting in uniform flowering and maturity. Hydrogen cyanamide is highly toxic, and adverse health effects from contact include severe irritation and ulceration of the eyes, skin, and respiratory tract (1,2). The substance also inhibits aldehyde dehydrogenase and can produce acetaldehyde syndrome (e.g., vomiting, parasympathetic hyperactivity, dyspnea, hypotension, and confusion) when exposure coincides with alcohol use. After Dormex® (Degussa AG, Trostberg, Germany), a pesticide product containing hydrogen cyanamide (49% by weight), was introduced in Italy in 2000, a total of 23 cases of acute illness associated with exposure to this chemical were identified in early 2001 (3). This led to a temporary suspension of sales and usage of Dormex on February 23, 2002, and strengthening of protective measures, as specified on the pesticide label when sales were resumed on June 20, 2003. This report describes 28 additional cases of hydrogen cyanamide--related illness that occurred during 2002--2004, 14 of which occurred after sales resumed. These illnesses suggest that the preventive measures adopted in Italy in 2003 to protect workers using hydrogen cyanamide are inadequate. Workers exposed to hydrogen cyanamide should be provided adequate information, training, personal protective equipment (PPE), and engineering controls. Public Health SurveillanceIn 2000, a pilot pesticide-poisoning surveillance program was undertaken by the Italian National Institute of Health (INIH) in collaboration with the Milan Poison Control Center (MPCC) and the Ragusa Local Health Unit (RLHU). This program has identified symptomatic cases involving Dormex exposure during 2001--2004. A previous report described cases from 2001 (3); cases identified during 2002--2004 are described in this report. Cases were classified as definite, probable, possible, or suspicious on the basis of clinical interpretation of signs or symptoms reported by a physician or patient and evidence of Dormex exposure (4). Illness severity§ was also categorized for all cases (5). A total of 28 hydrogen cyanamide--related illnesses were identified during 2002--2004. All cases were identified by MPCC, and three cases were detected by RLHU. Five cases were identified before the Dormex suspension was enacted, nine cases were identified during the suspension (whether workers used chemical purchased before the suspension or if an illegal purchase had occurred during the suspension is unknown), and 14 cases were identified after the suspension was lifted (Figure). All of the cases occurred in males; median age was 41 years (range: 25--65 years). All cases occurred from late December through early March of each year (Figure), which is the only period when Dormex is used in Italy. A total of 25 of the 28 cases occurred in persons who were exposed during application of Dormex. Another person was exposed while handling a Dormex packet found in the field and whose contents inadvertently spilled on the person, and one person was exposed from unintentional ingestion after the product was poured from its original container into a drinking bottle. For one case, no information on activity at time of exposure was available. Among the 25 cases involving exposure during application, 20 (80%) occurred in persons who were exposed while using a backpack sprayer, two (8%) while sitting in an open tractor cab, and one (4%) while crossing a treated field; information was not available for two cases (8%). Among the 21 cases with information available on use of PPE, only one involved a person who wore complete PPE (e.g., air-purifying respirator, goggles/face shield, chemical-resistant gloves, protective suit, and footwear). The majority (14 [66%]) of these persons used incomplete PPE (e.g., five reported using an air-purifying respirator but no other PPE), and six used no PPE. No deaths and no illnesses of high severity were identified. Eleven (40%) cases were classified as of moderate severity and 17 as low severity. Among the 14 cases that occurred after the Dormex suspension was lifted, seven (50%) were of moderate severity. The latency between exposure and onset of adverse effects ranged from 30 minutes to 30 hours. In 13 cases, signs or symptoms appeared immediately after alcohol consumption. Skin-related signs and symptoms occurred in most cases (Table). Reported by: L Settimi, PhD, I Marcello, National Institute of Health, Rome; F Davanzo, MD, L Faraoni, MD, Milan Poison Control Center, Cà Granda Hospital, Milan; G Miceli, MD, Occupational Health Svc, Ragusa Local Health Unit, Ragusa, Italy. D Richmond, California Dept of Pesticide Regulation. GM Calvert, MD, Div of Surveillance, Hazard Evaluation, and Field Studies, National Institute for Occupational Safety and Health, CDC. Editorial Note:The findings in this report indicate that, despite reports of hydrogen cyanamide--related cases in 2001 and strengthening of protective measures (i.e., requirements for more protective PPE and prohibition of some application methods) in Italy in 2003, illnesses related to hydrogen cyanamide continue to be identified. A comparison of the findings in Italy with case data in the United States might be useful. The United States has several surveillance systems to track pesticide poisoning, including the Sentinel Event Notification System for Occupational Risks (SENSOR)-Pesticides program, the California Department of Pesticide Regulation (CDPR) system, and the Toxic Exposure Surveillance System (4). During 2000--2004, only one U.S. case of hydrogen cyanamide--related illness was identified. This case was identified by CDPR and involved a worker in California in January 2004 who developed a rash on his wrists, arms, and knees within 24 hours of mixing and loading hydrogen cyanamide. Overall amounts of hydrogen cyanamide used in California are higher than in Italy. In 2001, an estimated 248,000 pounds of hydrogen cyanamide were used in California, compared with 80,000 pounds sold in Italy (6; Degussa AG, unpublished data, 2005), where it is primarily used on grapes. The number of illnesses identified since Dormex was reintroduced in Italy is lower than the number of illnesses observed in 2001 (14 from June 2003--December 2004 versus 23 in January--February 2001). Nevertheless, the number suggests that current preventive measures in Italy are not adequate. In particular, many workers did not use engineering controls or follow PPE requirements. Although the Italian pesticide label provides detailed information on PPE, the information on engineering controls is vague, with no mention of requiring closed systems. Efforts are needed to ensure the use of engineering controls (i.e., mixing, loading, and transferring of the chemical only in a closed system), which precludes the use of backpack sprayers. Of the 13 workers who became ill after the Dormex suspension was lifted, nine used backpack sprayers. Efforts are also needed to ensure appropriate use of PPE, which might require improved clarity of PPE information on the pesticide label and enhanced enforcement of PPE requirements. In addition, because many workers have onset of illness immediately after alcohol consumption, including eight workers after the Dormex suspension was lifted, the label language that warns against alcohol consumption might need to be strengthened. Because all of the cases were identified in three Italian regions (Table), these regions should be targeted with appropriate public health interventions. These interventions include educating growers and agricultural workers about hydrogen cyanamide--related health effects, ensuring that all exposed workers are provided with and trained to use appropriate PPE and promoting the adoption of engineering controls. As in other European countries, each pesticide product in Italy is assigned an overall risk category based on the toxicologic properties of the active ingredients in the product and their concentrations. When sales of Dormex were resumed in Italy in 2003, Italian authorities assigned a risk category of "toxic" to the product. Before Dormex sales were suspended in Italy in 2002, the product was assigned a risk category of "harmful," which is the equivalent of Environmental Protection Agency (EPA) toxicity category II¶. EPA classifies Dormex into the highest toxicity category (toxicity category I) because of its corrosive effects on the skin and eyes (7). EPA toxicity category I is equivalent to Italy's "toxic" category. In addition to the requirements imposed on all toxicity category I pesticides, EPA imposed additional restrictions to protect workers handling hydrogen cyanamide (e.g., consumption of alcoholic beverages is prohibited before, during, and 24 hours after handling this product). Hydrogen cyanamide is currently under regulatory review by European Union authorities (8). The findings in this report are subject to at least two limitations. First, the reported cases likely provide a minimum estimate of the true magnitude of hydrogen cyanamide--related illnesses in Italy. Although several poison control centers operate in Italy, only MPCC provided data for this report. However, MPCC is the most frequently consulted poison control center in Italy. Second, although all of these cases involved illnesses that were consistent with the case-definition criteria, the possibility of false positives cannot be excluded. Given the nonspecificity of the clinical findings of hydrogen cyanamide poisoning and the lack of a "gold standard" diagnostic test, some illnesses temporally related to hydrogen cyanamide exposure might be coincidental and not exposure related. The findings in this report demonstrate the value of using surveillance data to evaluate the effectiveness of an intervention. In addition, the findings illustrate the international nature of pesticide-related concerns and the usefulness of coordination of policies and requirements to protect worker health and safety. References
* Chemical Abstracts Service no. 420-04-2. The entire case definition is available at http://www.cdc.gov/niosh/topics/pesticides/pdfs/casedef2003_revAPR2005.pdf. § A low severity illness consists of minimally bothersome health effects that generally resolve rapidly. A moderate severity illness or injury consists of non--life-threatening health effects that are more pronounced, prolonged, or of a systemic nature, compared with a low severity illness. A high severity illness or injury consists of life-threatening health effects or those that result in substantial residual disability or disfigurement. ¶ EPA classifies all pesticide products into one of four toxicity categories on the basis of established criteria (40 CFR 156). Pesticides with the greatest toxicity are in category I, and those with the least are in category IV. Table 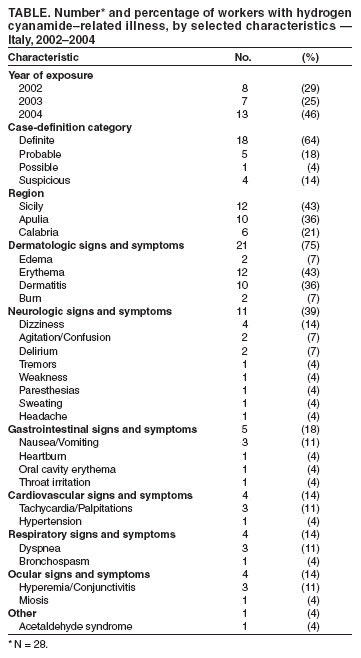 Return to top. Figure 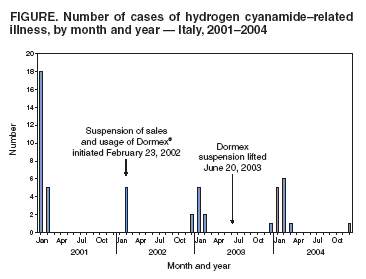 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 4/28/2005 |
|||||||||
|