 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Outbreak of Cyclosporiasis Associated with Snow Peas --- Pennsylvania, 2004On September 17, this report was posted as an MMWR Early Release on the MMWR website (http://www.cdc.gov/mmwr). During June--July 2004, public health officials in Pennsylvania were notified of cases of the parasitic disease cyclosporiasis (1,2) among persons associated with a residential facility (e.g., residents, staff, and volunteers). CDC confirmed the diagnosis of Cyclospora cayetanensis infection (1) by examining stool specimens from multiple patients. By early July, local public health officials had been notified of approximately 50 potential cases of cyclosporiasis associated with the facility; onsets of illness were from early June through early July. This report describes the findings of the epidemiologic and traceback investigations, which determined the cases were linked to consumption of raw Guatemalan snow peas at five special events, for which food was prepared by the facility staff, from late May through late June (Table). This is the first documented outbreak of cyclosporiasis linked to snow peas. The Food and Drug Administration (FDA) and CDC are working with Guatemalan officials to determine the sources of the snow peas and possible modes of contamination. A case of cyclosporiasis was defined as onset of illness 1--14 days after consumption of food or beverages served at one or more of the five special events. Persons with laboratory-confirmed cases had infection confirmed by CDC by examining stool specimens for Cyclospora (1), and at least one gastrointestinal (GI) symptom (i.e., diarrhea [loose or watery stool], nausea, vomiting, abdominal cramps, loss of appetite, or unintentional weight loss) or constitutional symptom (i.e., fever, chills, muscle aches, joint aches, generalized body aches, headache, or fatigue). Persons with probable (clinically defined) cases of infection either had 1) three or more loose or watery stools during a 24-hour period and at least one other symptom or 2) five or more symptoms, including at least three GI symptoms. Of the 349 persons associated with the facility who were in the population potentially at risk for infection, 315 (90%) persons were interviewed to ascertain exposure (e.g., event attendance) and illness status; 215 (68%) of the 315 interviewed had attended at least one event. Of the 215 persons, 96 (45%) had illness that was consistent with one of the case definitions; 40 cases were laboratory confirmed, and 56 were probable. All of the cases were associated with special events (i.e., none were attributable to other meals at the facility, which were prepared by the same staff and in the same kitchen), and each of the five events was associated with laboratory-confirmed cases. Therefore, the investigation focused on identifying an item served at all five events, but not at other meals. Only pasta salad met these criteria. In addition, pasta salad was the only food item statistically significantly associated with illness in retrospective cohort studies, which were conducted among persons who attended the two most recent events (events D and E) (Table); data for 77 attendees were included in analyses. The summary relative risk for these two events (i.e., for the association between pasta salad and illness) was 32 (95% confidence interval: 5--219; p<0.001). Specifically, 90% (38 of 42) of the persons who ate the salad had cases of cyclosporiasis, compared with 3% (one of 35) of the persons who did not eat the salad. The median incubation periods for illness associated with events D and E were 8 days (range: 1--13 days) and 7 days (range: 1--10 days), respectively. The pasta salad included multiple types of raw produce, none of which were implicated in investigations of previous outbreaks of cyclosporiasis (1). Of the produce used in the salad (Table), only snow peas met all of the following criteria: 1) were included in all three batches of the salad served at the five events, 2) were from the same "lot" (i.e., from one container, purchased on 1 day, and from one supplier), and 3) were not served at other meals except the five events. Event A on May 31 (Table) was the first occasion in 2004 at which pasta salad or snow peas were served by the facility. All of the snow peas used by the facility came from the same 4.5-kg container, which was purchased on May 21 and refrigerated thereafter. On June 22, after the last (third) batch of salad was prepared (Table), the residual snow peas were discarded; none were available for testing for Cyclospora oocysts or DNA when the investigation was initiated. In an investigation conducted by FDA, the snow peas were traced to an exporter in Guatemala. The snow peas were handled only on days when batches of salad were prepared (Table). For each batch, a handful of peas (approximately 1 kg) was removed from the container, washed in municipal water, and added to the salad. None of the food handlers who prepared or served the salad had symptoms consistent with cyclosporiasis before the onset of the outbreak or on the days the first two batches were prepared or served (Table). One person who helped prepare the third batch had a probable case of cyclosporiasis after eating salad from the first two batches. Reported by: A Crist, PhD, York Hospital, York; C Morningstar, R Chambers, T Fitzgerald, D Stoops, M Deffley, Y Reyes, T Hiden, J Sullivan, D Hawk, MD, York City Health Bur; P Lurie, MD, M Moll, MD, Div Infectious Disease Epidemiology; S Yeager, L Lind, MPH, J Burkee, K Warren, MPH, M Marcus, J Reeser, H Davidson, S Thomas, Bur of Community Health Systems, Pennsylvania Dept of Health. Food and Drug Administration, College Park and Rockville, Maryland. BL Herwaldt, MD, M Hlavsa, MPH, SP Johnston, MS, H Bishop, A daSilva, PhD, A Hightower, MS, Div Parasitic Diseases, National Center for Infectious Diseases; DK El Reda, DrPH, N Flowers, MD, EIS officers, CDC. Editorial Note:The findings of this investigation indicate that raw Guatemalan snow peas were linked to this outbreak of cyclosporiasis in Pennsylvania. No evidence of ongoing transmission has been obtained, despite heightened surveillance for cases of cyclosporiasis. This is the first investigation in which snow peas have been implicated as the vehicle of an outbreak of cyclosporiasis. Several other types of fresh produce (e.g., raspberries, basil, and mesclun lettuce), from various countries, have previously been implicated as vehicles of U.S. cyclosporiasis outbreaks (1). FDA and CDC are working with Guatemalan officials to determine the sources of the snow peas (e.g., farms or cooperatives) and possible modes of contamination. The modes of contamination of implicated vehicles have not been definitively determined for any previous foodborne outbreak of cyclosporiasis (1); better understanding of the biology and epidemiology of the parasite is needed. For imported vehicles of infection, international collaboration is critical to the success of investigations and to the identification of appropriate prevention and control measures. Produce should be thoroughly washed before it is eaten. This practice might decrease but not eliminate the risk for transmission of Cyclospora (1,3). Health-care providers should consider the diagnosis of Cyclospora infection in persons with prolonged or remitting-relapsing diarrheal illness and specifically request testing of stool specimens for this parasite (1); such testing is not routinely conducted by most laboratories. Trimethoprim/sulfamethoxazole (TMP/SMX) has been shown in a placebo-controlled trial to be effective treatment for Cyclospora infection (4). Adults should receive TMP 160 mg plus SMX 800 mg (one double-strength tablet) orally, twice a day for 7 days. Some patients might benefit from longer courses of therapy. Alternative treatments for persons allergic to sulfa drugs have not yet been identified (1). Newly identified clusters of cases of cyclosporiasis should be investigated to identify the vehicles of infection and their sources and modes of contamination. Although cases of cyclosporiasis are not yet reportable in all U.S. states and territories, such cases are nationally notifiable. Clinicians and laboratorians who identify cases of cyclosporiasis, especially ones unrelated to foreign travel, are encouraged to inform the appropriate local public health officials, who are encouraged to contact CDC's Division of Parasitic Diseases, National Center for Infectious Diseases. To report cases and potential outbreaks, contact CDC, telephone 770-488-7319; for questions about laboratory diagnosis of infection, 770-488-4474; for clinical questions, 770-488-7775. Additional information about cyclosporiasis is available at http://www.cdc.gov/ncidod/dpd/parasites/cyclospora/default.htm. Acknowledgments This report is based in part on contributions by W Ness, MBA, D German, S Leyland, community partners, Pennsylvania. R Klein, PhD, Medical Entomology Research and Training Unit/Guatemala City, Guatemala; C Bern, MD, M Bartlett, Div Parasitic Diseases, National Center for Infectious Diseases, CDC. References
Table 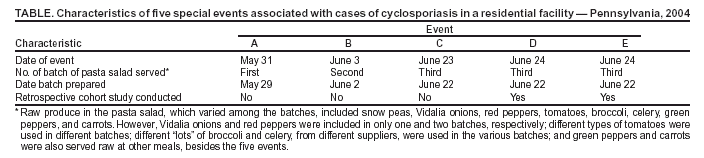 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/23/2004 |
|||||||||
This page last reviewed 9/23/2004
|