 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Measles Outbreak in a Boarding School --- Pennsylvania, 2003Measles has not been endemic in the United States since 1997, although limited outbreaks continue to be caused by imported* cases (1,2). In 2003, CDC assisted in investigating the largest school outbreak of measles in the United States since 1998 (3). The outbreak consisted of 11 laboratory-confirmed cases: nine cases in a boarding school in eastern Pennsylvania and two epidemiologically linked cases in New York City (NYC). This report summarizes the results of the outbreak investigation, which indicated that measles continues to be imported into the United States and that high coverage with 2 doses of measles-containing vaccine (MCV) among students was effective in limiting the size of the outbreak. Health-care providers should maintain a high index of suspicion for measles, especially in those who have traveled abroad recently, and recommendations for 2 doses of MCV in all school-aged children should be followed. In April 2003, the Pennsylvania Department of Health reported to CDC two cases of measles in unvaccinated twins aged 13 years in a boarding school with 663 students. Active surveillance for measles was conducted in the school, hospitals, and doctors' offices through May 2003. Patients were interviewed, acute- and convalescent-phase sera were collected for measles IgM enzyme-linked immunosorbent assay testing, and throat swabs and urine samples were collected for viral genotyping. Efforts to control the outbreak included vaccinating or excluding from campus and isolating all students and staff members with no evidence of immunity to measles§. School and personal vaccination records were reviewed to identify susceptible students and staff members, respectively. For evaluation of vaccine effectiveness, only students enrolled in the school at the beginning of the outbreak were included. All staff members and those students who received measles vaccination during the outbreak were excluded. Vaccine effectiveness (VE) was calculated as VE (%) = [(ARU - ARV) / ARU] x 100, where ARU is the attack rate in unvaccinated persons and ARV is the attack rate in students who had received 2 doses of MCV previously (4). A total of 11 laboratory-confirmed cases of measles were identified. The source patient was a student aged 17 years who had received 2 doses of MCV. On March 15, 2003, the student had returned to the United States from Beirut, Lebanon, where measles was known to be circulating. He had cough and fever the following day and rash on March 21, when he visited an emergency department and was diagnosed with a viral exanthem. Upon returning to school, the patient stayed at the school health center before returning to his dormitory. Five persons with laboratory-confirmed measles were linked epidemiologically to the source patient. These five included the unvaccinated twins who lived in the same dormitory, the dormitory houseparent, and two other students in different dormitories. One of these latter students infected two additional students in his dormitory and an unvaccinated child aged 13 months in NYC, who was linked epidemiologically to an unvaccinated immigrant aged 33 years, who was diagnosed with measles and who lived in the same apartment building. The ninth school patient was linked epidemiologically to, and might have been infected by, any one of five infected persons from different dormitories. All nine measles cases in the school were confirmed serologically. Measles genotype D4 was identified in two school patients and the child in NYC. The last date of rash onset in a boarding school patient was April 15 (Figure). No deaths or major complications were reported; two students with measles, who were unvaccinated because of religious exemptions, required hospitalization for dehydration. The median age of the nine patients in the school was 17 years (range: 13--26 years). Of the nine, two had not received any doses of MCV, one had received 1 dose, and six had received 2 doses. Patients with 1 or 2 doses of MCV had milder illness than unvaccinated patients, including a shorter duration of rash (median: 5 days versus 10 days; p<0.05) and fewer days of school or work missed (median: 5 days versus 8 days; p<0.05) (Table). Of the 663 students in the boarding school, eight (1.2%) students had never received any doses of MCV, 26 (3.9%) students had received 1 dose, and 629 (94.9%) students had received 2 doses before the outbreak. Thus, vaccine coverage for 2 doses was 94.9% and for >1 dose was 98.8%. Vaccination with measles, mumps, and rubella vaccine was begun on April 3. Of the eight unvaccinated students, four had claimed religious or philosophical exemptions. Of these four students, two contracted measles, one was excluded from the school, and one was vaccinated during the outbreak. All of the remaining four unvaccinated students who did not claim any exemptions were vaccinated during the outbreak as well as other susceptible students and staff members. Excluding five previously unvaccinated students who were vaccinated during the outbreak and two students who had 2 doses of MCV previously but were inadvertently revaccinated during the outbreak, the measles attack rate was 66.7% (two of three) among unvaccinated students and 1.0% (six of 627) among students who had received 2 doses of MCV. All vaccinees with 1 dose of MCV received a second dose during the outbreak; no measles cases were diagnosed among these students. VE was 98.6% among students who had received 2 doses of MCV. Reported by: P Lurie, MD, P Britz, J Bowen, P Tran, MEd, H Stafford, Pennsylvania Dept of Health. YA Gillan, DrPH, New York City Dept of Health and Mental Hygiene. W Bellini, PhD, P Rota, PhD, J Rota, MPH, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; E Eduardo, MPH, National Center for HIV, STD, and TB Prevention; G Dayan, MD, S Redd, M Papania, MD, J Seward, MBBS, Epidemiology and Surveillance Div, National Immunization Program; RJ Berry, MD, Birth Defects and Developmental Disabilities Div, National Center on Birth Defects and Developmental Disabilities; L Yeung, MD, EIS Officer, CDC. Editorial Note:Measles is rare in the United States, with only 42 confirmed cases in 2003, according to provisional data (2). The limited outbreak described in this report highlights both the success of the U.S. vaccination program and the continuing risk for imported measles despite a high immunity among the U.S. population. The last reported U.S. school outbreak occurred in 2000 and involved nine persons, including six high school students (1). Five of those six student patients had received only 1 dose of MCV, which was in compliance with state requirements at that time (1). Before 1989, when the Advisory Committee on Immunization Practices recommended a routine 2-dose MCV schedule for school-aged children, larger measles outbreaks with >100 cases occurred in schools (5,6). All states but one now require 2 doses of MCV for children attending school (7). However, exemptions for religious or philosophical reasons are permitted in the majority of states, resulting in exemption for 0.6% of the nation's children (8). These children have a higher likelihood of acquiring and spreading measles than those who have been vaccinated (9). In the outbreak described in this report, consistent with previous evaluations (10), 2 doses of MCV were highly effective in preventing the spread of measles, although a substantial number of exposed students, combined with a 1% failure rate among recipients with 2 doses, resulted in two generations of transmission in the school. Recipients of 2 doses of MCV had milder symptoms and shorter duration of illness than unvaccinated patients. Two unvaccinated students were hospitalized for dehydration, but none of the vaccinated students required hospitalization. If an outbreak occurs, all persons whose illness is consistent with the definition for suspected¶ measles should be tested for both measles IgM and measles virus by culture or reverse transcriptase polymerase chain reaction. A convalescent serum should be obtained if the acute IgM is negative. This investigation highlighted the importance of viral specimens to document importation from overseas, confirm spread of the same genotype to NYC, and provide continued evidence for the absence of endemic transmission in the United States (1). This outbreak of measles was caused by importation; the source patient was infected in Lebanon. Although the patient had classic signs for the disease (e.g., fever, rash, cough, and coryza), measles was not diagnosed initially, and the outbreak was not recognized until two unvaccinated students were hospitalized. A history of recent travel outside the United States should raise suspicion for a diagnosis of measles in a patient with appropriate clinical signs, regardless of vaccination status. References
* An imported case of measles has its source outside the country, rash onset occurs within 21 days of entering the country, and illness cannot be linked to local transmission. Surveillance was conducted by using the 1997 case definition for measles issued by CDC and the Council of State and Territorial Epidemiologists: illness characterized by a generalized maculopapular rash lasting >3 days; a temperature of >101.0º F (>38.3º C); and cough, coryza, or conjunctivitis. § Students and staff members were classified as having no evidence of measles immunity if they were born after 1957 and could not document history of physician-diagnosed measles illness, positive serology of measles IgG, history of 2 doses of MCV at least 28 days apart (students), or 1 dose (adults) with the first dose at or after age 1 year. ¶ Suspected measles is a febrile illness with a generalized maculopapular rash. Table 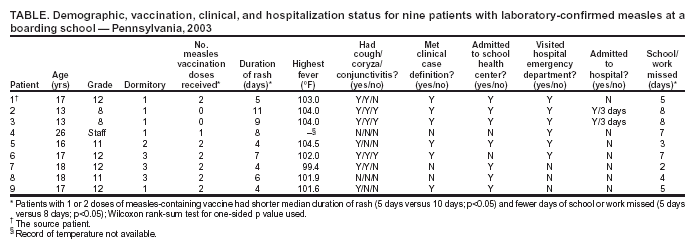 Return to top. Figure 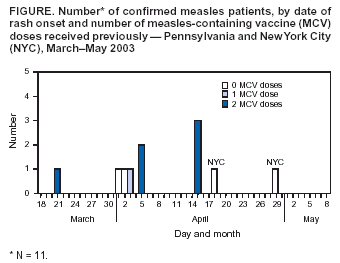 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
|
|||||||||
This page last reviewed 4/15/2004
|