 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Cases of Influenza A (H5N1) --- Thailand, 2004Since mid-December 2003, eight Asian countries (Cambodia, China, Indonesia, Japan, Laos, South Korea, Thailand, and Vietnam) have reported an epizootic of highly pathogenic avian influenza in poultry and various other birds caused by influenza A (H5N1). As of February 9, 2004, a total of 23 laboratory-confirmed human cases of influenza A (H5N1) had been reported in Thailand and Vietnam. In 18 (78%) of these cases, the patients died. Clinical experience with avian H5N1 disease in humans is limited (1). The human H5N1 viruses identified in Asia in 2004 are antigenically and genetically distinguishable from the 1997 and February 2003 viruses. To aid surveillance and clinical activities, this report provides a preliminary clinical description of the initial five confirmed cases in Thailand. Of the five laboratory-confirmed cases in Thailand, four were in male children aged 6--7 years, and one was in a female aged 58 years; all patients were previously healthy (Table). Four patients reported deaths in poultry owned by the patient's family, and two patients reported touching an infected chicken. One patient had infected chickens in his neighborhood and was reported to have played near a chicken cage. None of the confirmed cases occurred among persons involved in the mass culling of chickens. Patients reported to hospitals 2--6 days after onset of fever and cough (Table). Other early symptoms included sore throat (four), rhinorrhea (two), and myalgia (two). Shortness of breath was reported in all patients 1--5 days after symptom onset. On admission, clinically apparent pneumonia with chest radiograph changes was observed in all patients, with patchy infiltrates in four and interstitial infiltrates in one. Diarrhea and vomiting were not reported. Peripheral leukocytes were normal or decreased, and four patients had lymphopenia (<1,000/µL). Mild-to-moderate elevations in hepatic transaminases were found in four patients. All patients had respiratory failure and required intubation a median of 7 days (range: 4--10 days) after onset of illness. Two patients had a pneumothorax. Three patients required inotropic support for decreased cardiac function; two patients had renal impairment as a later manifestation. None had documented evidence of secondary bacterial infection. Late in the course of illness, three patients were treated with oseltamivir for 3--5 days. All received empiric broad-spectrum antibiotics for community-acquired pneumonia while the cause of illness was under investigation. Four were treated with systemic steroids for increasing respiratory distress and clinically diagnosed acute respiratory distress syndrome (ARDS) with compatible chest radiograph changes. Three children died 2--4 weeks after symptom onset, and one child and the adult died 8 days after symptom onset. All patients had laboratory evidence of influenza A (H5N1) by reverse transcriptase--polymerase chain reaction. In three cases, the virus was isolated in tissue culture, and in three cases, the viral antigens were identified by immunofluorescent assay. Reported by: T Chotpitayasunondh, S Lochindarat, P Srisan, Queen Sirikit National Institute of Child Health; K Chokepaibulkit, Faculty of Medicine, Siriraj Hospital, Mahidol Univ, Bangkok; J Weerakul, Buddhachinaraj Hospital, Phitsanulok; M Maneerattanaporn, 17th Somdejprasangkaraj Hospital, Suphanburi; P Sawanpanyalert, Dept of Medical Sciences, Ministry of Public Health, Thailand. World Health Organization, Thailand. CDC International Emerging Infections Program, Thailand. Editorial Note:The 1997 outbreak of influenza A (H5N1) in Hong Kong established that highly pathogenic avian influenza viruses can infect humans directly, with resulting illness that was fatal in six (33%) of 18 patients. The viruses were not transmitted efficiently from person to person, and human infections stopped after the culling of poultry (2). The 2003--2004 avian outbreak is more widespread, with poultry disease reported across much of east and southeast Asia. Direct infection of humans with H5N1 viruses has been confirmed in Thailand and Vietnam. However, no evidence of sustained person-to-person transmission has been identified. Despite the antigenic and genetic differences in the H5N1 viruses causing the current Asian outbreaks, certain clinical features of the five human cases described in this report are similar to those of severely affected patients from the 1997 outbreak in Hong Kong (3). In all five cases, disease was severe, with pneumonia progressing to respiratory failure and death. Early distinguishing features included fever, sore throat, cough, and lymphopenia. Other organ involvement included mild-to-moderate hepatitis and later cardiac and renal impairment. In contrast with the cases reported from Hong Kong, gastrointestinal symptoms were not prominent features. Because of the severity of disease and the concern for the safety of health-care personnel, the Ministry of Public Health in Thailand recommends that hospitalized patients with suspected avian influenza be cared for by using precautions to minimize the risk for airborne transmission. Broad-spectrum antibacterial drugs should be used as empiric treatment for the major causes of pneumonia (e.g., Streptococcus pneumoniae), including possible superinfection with Staphylococcus aureus. Testing of a limited number of human isolates demonstrates resistance to amantadine and rimantadine (4). For this reason, treatment with neuraminidase inhibitors should be initiated early. The effectiveness of antiviral drugs against H5N1 infections and the period after which these drugs will provide little or no benefit is not known. A more detailed understanding of the pathogenesis is needed to direct therapeutic approaches such as the use of immunomodulating drugs. Updated recommendations for hospital infection control and treatment are available from the World Health Organization at http://www.who.int/csr/disease/avian_influenza/en. The epidemiology of influenza A (H5N1) in Thailand and neighboring countries remains incompletely described, but the confirmed human infections have occurred in geographic areas with recognized avian disease, and two patients reported direct physical contact with ill or dead chickens. Of the five laboratory-confirmed cases in Thailand, four were in boys aged 6--7 years, which suggests that boys in this age group might be subject to particular high-risk exposures. Case-control studies in Thailand and Vietnam should help define specific risk factors for infection and allow for the development of evidence-based public health interventions. Control of highly pathogenic avian influenza should include surveillance for affected flocks, aggressive culling on the basis of international guidelines to eradicate foci of infection, careful protection of cullers through the use of personal protective equipment, and use of the currently licensed human trivalent influenza vaccine to reduce the risk for co-infection in poultry workers and cullers, which might lead to genetic reassortment of avian and human influenza viruses (2,3). In recent weeks, Thailand has moved aggressively to 1) identify geographic areas with confirmed H5N1 disease in poultry (e.g., cull-affected flocks and flocks within a 5-kilometer radius), 2) establish controls on the transport of poultry and poultry products out of affected areas, and 3) promote safe food-handling practices. Clinicians should be aware of the clinical features of the current human influenza A (H5N1) disease and the potential risk factors for infection so that health-care workers are protected and patients can be identified quickly and managed appropriately. Interim U.S. recommendations for infection-control precautions and the diagnostic evaluation of persons with specific epidemiologic and clinical criteria have been developed (4). Additional information is available from CDC at http://www.cdc.gov/flu/avian/index.htm. References
Table 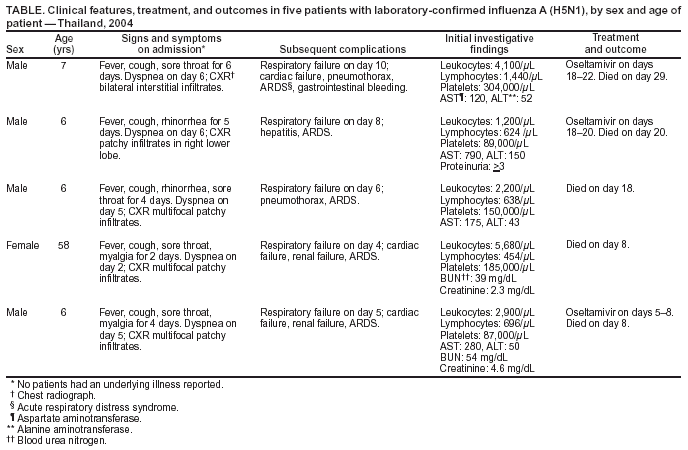 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/12/2004 |
|||||||||
This page last reviewed 2/12/2004
|