 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Updated Interim Surveillance Case Definition for Severe Acute Respiratory Syndrome (SARS) --- United States, April 29, 2003CDC's interim surveillance case definition for severe acute respiratory syndrome (SARS) has been updated to include laboratory criteria for evidence of infection with the SARS-associated coronavirus (SARS-CoV) (Figure, Box). In addition, clinical criteria have been revised to reflect the possible spectrum of respiratory illness associated with SARS-CoV. Epidemiologic criteria have been retained. The majority of U.S. cases of SARS continue to be associated with travel*, with only limited secondary spread to household members or health-care providers (1). SARS has been associated etiologically with a novel coronavirus, SARS-CoV (2,3). Evidence of SARS-CoV infection has been identified in patients with SARS in several countries, including the United States. Several new laboratory tests can be used to detect SARS-CoV. Serologic testing for coronavirus antibody can be performed by using indirect fluorescent antibody or enzyme-linked immunosorbent assays that are specific for antibody produced after infection. Although some patients have detectable coronavirus antibody during the acute phase (i.e., within 14 days of illness onset), definitive interpretation of negative coronavirus antibody tests is possible only for specimens obtained >21 days after onset of symptoms. A reverse transcriptase polymerase chain reaction (RT-PCR) test specific for viral RNA has been positive within the first 10 days after onset of fever in specimens from some SARS patients, but the duration of detectable viremia or viral shedding is unknown. RT-PCR testing can detect SARS-CoV in clinical specimens, including serum, stool, and nasal secretions. Finally, viral culture and isolation have both been used to detect SARS-CoV. Absence of SARS-CoV antibody in serum obtained <21 days after illness onset, a negative PCR test, or a negative viral culture does not exclude coronavirus infection. Reported U.S. cases of SARS still will be classified as suspect or probable; however, these cases can be further classified as laboratory-confirmed or -negative if laboratory data are available and complete, or as laboratory-indeterminate if specimens are not available or testing is incomplete. Obtaining convalescent serum samples to make a final determination about infection with SARS-CoV is critical. No instances of SARS-CoV infection have been detected in persons who are asymptomatic. However, data are insufficient to exclude the possibility of asymptomatic infection with SARS-CoV and the possibility that such persons can transmit the virus. Investigations of close contacts and health-care workers exposed to SARS patients might provide information about the occurrence of asymptomatic infected persons. Similarly, the clinical manifestations of SARS might extend beyond respiratory illness. As more is learned about SARS-CoV infection, clinical and laboratory criteria will provide a framework for classifying the full spectrum of infection (Figure). This surveillance case definition should be used for reporting and classification purposes only. It should not be used for clinical management or as the only criterion for identifying or testing patients who might have SARS or for instituting infection-control precautions (4,5). This definition will be updated as new data become available or if changes in the epidemiology of SARS occur in the United States. References
* In this updated case definition, Taiwan has been added to the areas with documented or suspected community transmission of SARS; Hanoi, Vietnam is now an area with recently documented or suspected community transmission of SARS. Box Return to top. Figure 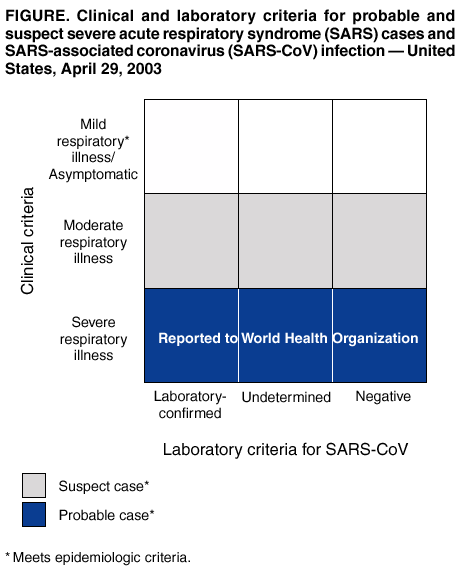 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/30/2003 |
|||||||||
This page last reviewed 4/30/2003
|