 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Pregnancy in Perinatally HIV-Infected Adolescents and Young Adults --- Puerto Rico, 2002Please note: An erratum has been published for this article. To view the erratum, please click here. Since the introduction of highly active antiretroviral (ARV) therapy in the United States in the mid-1990s (1--3), the life expectancy of U.S. children who were infected perinatally with human immunodeficiency virus (HIV) has increased substantially. As a result, the number of perinatally HIV-infected females in the United States who are becoming both sexually active and pregnant is increasing (4). During August 1998--May 2002, a total of 10 pregnancies were identified among eight perinatally HIV-infected adolescents and young adults in Puerto Rico; in April 2002, the Puerto Rico Department of Health (PRDOH) asked CDC to assist in assessing such pregnancies. This report describes these pregnancies and discusses factors associated with sexual activity and pregnancy. The findings suggest that increasing numbers of pregnancies will occur among perinatally HIV-infected adolescents and young adults and that appropriately tailored reproductive health interventions should be developed. Adolescents and young adults were identified by their health-care providers or by PRDOH, and chart reviews and interviews were conducted during April--August 2002. For females with two pregnancies, interview and chart data on the first pregnancy are reported. Case-patients were defined as perinatally HIV-infected adolescents or young adults with a history of pregnancy, and controls were defined as perinatally HIV-infected females with no history of pregnancy. All controls were age-matched to <1 year of the age of the pregnant females, except for one patient aged 22 years who had been aged 19 years when she was pregnant; she was matched to a control aged 19 years. Perinatal infection was defined as confirmed HIV-positive serostatus of the patient's biologic mother or an HIV risk factor for the biologic mother and the absence of any other risk factors (e.g., sexual abuse or blood transfusions) for the patient. A total of eight case-patients were identified in four cities in Puerto Rico. The median age of the case-patients was 18 years (range: 15--22 years), and the median age at the time of first pregnancy was 17 years (range: 13--19 years). Among the 10 pregnancies to the eight patients, seven pregnancies in six patients resulted in live-born infants; as of February 24, no cases of mother-to-child HIV transmission were reported. In addition, two pregnancies ended in elective abortions and one in a spontaneous abortion. Five case-patients had first pregnancies that resulted in live-born infants; all five received some prenatal care, and four (80%) received ARV therapy consistently during their pregnancies. All infants received zidovudine prophylaxis after delivery. The median viral load of these case-patients during pregnancy was 35,822 copies/mL (range: 3,535--163,064 copies/mL), and the median CD4 count during pregnancy was 218 cells/mm3 (range: 19--956 cells/mm3). The majority of the case-patients were highly ARV-experienced, with a median of >9 years (range: 3--12 years) of ARV therapy, and five case-patients had each taken at least nine different ARV medications during their lifetimes. All four case-patients who were tested for viral resistance had multiple genotypic mutations. Five of the eight case-patients reported unintended pregnancies, and two reported using condoms as a form of birth control at the time they conceived. Six case-patients are now living with partners; one is in school, two left school because they were pregnant, and five left for reasons other than pregnancy or motherhood. Eight controls were included in the analysis. The median age of case-patients and of controls at the time they were interviewed was 18 years (range: 15--22 years) and 17 years (range: 14--19 years), respectively. The median age of HIV diagnosis was 7 years (range: 0--13 years) for case-patients and 4 years (range: 2--13 years) for controls. Differences in clinical outcomes included a median viral load since 1999 of 16,263 copies/mL (range: 5,251--65,571 copies/mL) for case-patients and of 53,071 copies/mL (range: 54--476,139 copies/mL) for controls and a median CD4 count since 1999 of 251 cells/mm3 (range: 72--1,296 cells/mm3) for case-patients and of 293 cells/mm3 (range: 66--1,002 cells/mm3) for controls. Behavioral and social characteristics associated with sexual activity and pregnancy were compared for all 16 case-patients and controls; all eight case-patients and two controls who reported being active sexually were asked questions about sexual activity. More case-patients than controls had dropped out of school before pregnancy and had friends who had become pregnant before they did (Table). The mean age when they were first told their HIV status was 13 years (range: 12--15 years) for case-patients and 12 years (range: 8--14 years) for controls. The median age at first sexual activity was 15 years (range: 13--18 years) for case-patients and 17 years (range: 15--18 years) for controls. The median time that elapsed between being told their HIV status and becoming sexually active was 2 years (range: 0--5 years) for case-patients and 5 years (range: 4--6 years) for controls. Three case-patients and no controls became sexually active at the same age that they were first told their HIV status. Case-patients and controls were asked about their counseling needs with respect to sexual activity, pregnancy, and birth control, and case-patients were asked about discussions of sexual activity, pregnancy, and birth control before their pregnancies. Two case-patients and five controls reported having discussed sexual activity, pregnancy, or birth control with a family member. Of all 16 persons surveyed, 10 wanted more reproductive health information, 10 believed that health-care providers were an important source of reproductive health information, and eight believed that families and schools should discuss these topics. Reported by: C Zorrilla, MD, I Febo, MD, Univ of Puerto Rico, San Juan; I Ortiz, MD, JC Orengo, MD, S Miranda, MPH, M Santiago, MPH, A Rodriguez, MD, J Rullan, MD, Puerto Rico Dept of Health. K Dominguez, MD, MG Fowler, MD, A Greenberg, MD, Div of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention; M McConnell, MD, EIS Officer, CDC. Editorial Note:This report describes pregnancies in perinatally HIV-infected adolescents and young adults for the first time and highlights the challenges in developing appropriately tailored reproductive health services for this growing population in the United States. During the early 1980s, when the first perinatally acquired AIDS cases were documented, infection in the majority of children progressed rapidly to death. Therefore, these children were not expected to survive to adolescence and mature to become sexually active. The findings of this investigation suggest that the risk-taking sexual behaviors of perinatally HIV-infected adolescents and young adults might not differ from those of non-HIV-infected adolescents and young adults (5,6). Although ARV therapy has made perinatal HIV transmission in this population infrequent in the United States (7), as the perinatally HIV-infected population ages, increasing numbers of pregnancies in perinatally HIV-infected female adolescents and young adults can be anticipated, and reproductive health issues affecting this population will need to be addressed. Factors that might be associated with pregnancy in these females include a relatively late age at disclosure of HIV status and inconsistent condom use with sex partners. These findings underscore the need for early disclosure of HIV status to infected adolescents and young adults and for increased discussions about sexual risk reduction among all perinatally infected adolescents and young adults. Providing families with the tools for HIV disclosure to children and for reproductive health discussions before sexual initiation might reduce risky behaviors among these females. The findings in this report are subject to at least two limitations. First, the small sample size makes the findings largely descriptive. Second, matching by age might not reflect social or physical development. Both of these limitations reduce the degree to which generalizations can be based on the data. Enhanced efforts to identify pregnancies among perinatally HIV-infected adolescents and young adults and more in-depth investigation of such pregnancies could better characterize the factors associated with pregnancies and birth outcomes. The finding of genotypic mutations of HIV isolated in all persons tested in Puerto Rico reinforces the importance of preventing secondary HIV transmission both to infants and sex partners. Surveillance of birth outcomes in perinatally HIV-infected adolescents and young adults and of cases of mother-to-child transmission and transmission of drug-resistant virus should continue. To permit accurate monitoring of trends in HIV transmission, clinicians should report births to HIV-infected women and adolescents to their health departments according to state surveillance guidelines for HIV/AIDS reporting. In addition, to assist CDC with determining pregnancy outcomes among this population, clinicians are urged to report pregnancies among perinatally HIV-infected adolescents and young adults directly to CDC, telephone, 404-639-6141, or e-mail, mmcconnell@cdc.gov, through June 2003. Acknowledgments This report is based on data contributed by L Ortiz, Univ of Puerto Rico, San Juan; L Pena, O Garcia, MD, Pediatric Immunology Clinic, Bayamon; D Padilla, R Delgado, MD, Center for Prevention and Treatment of Transmissible Diseases, Ponce; A Negron, M de los Angeles del Rio, MD, Center for Prevention and Treatment of Transmissible Diseases, Mayaguez; E Perez, R Jimenez, Puerto Rico Dept of Health. B Bohannon, Northrup Grumman Mission System, Atlanta, Georgia. References
Table 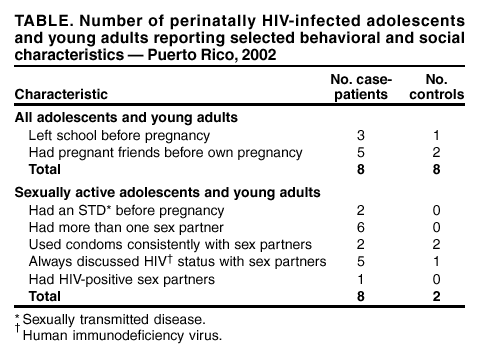 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 2/27/2003 |
|||||||||
This page last reviewed 2/27/2003
|