 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Nationwide Campaign for Vaccination of Adults Against Rubella and Measles --- Costa Rica, 2001In 1999 in Costa Rica, a large rubella outbreak occurred among persons aged 15--45 years. In response, the Ministry of Health adopted the goal of eliminating rubella and congenital rubella syndrome (CRS). In 2001, a nationwide vaccination campaign reached approximately 1.6 million (>95%) persons aged 15--39 years. This report highlights successful aspects of the campaign, including effective planning, cooperation among government ministries, social mobilization, the use of house-to-house vaccination teams, daily coverage reports from local staff, vaccine safety monitoring, and strategies for ensuring a sufficient national blood supply. This campaign will strengthen measles eradication and lead to rubella and CRS elimination in Costa Rica. In Costa Rica, measles vaccine was introduced in 1967, the combined measles-rubella (MR) vaccine in 1972, and measles-mumps-rubella (MMR) in 1986 as a single dose for children at age 12 months. Since 1992, a second dose of MMR vaccine has been recommended for children aged 7 years, and nationwide campaigns were conducted in 1992 (targeting children aged 1--4 years), 1997 (targeting children aged 1--14 years), and 1999 (targeting children aged 7--14 years) (Figure 1) (1). In 1996, a nationwide serosurvey indicated that rubella immunity was lowest (46%) among persons aged 15--24 years (2). In 1999, a rubella outbreak, in which 906 (84%) of 1,083 cases occurred among persons aged 15--45 years, prompted an MMR campaign among children aged 7--14 years (Figure 2). On the basis of age-specific data on the incidence of rubella, age-specific fertility rates, and the risk for CRS during pregnancy, 30 CRS cases were projected to occur following the 1999 outbreak. In response, the Ministry of Health implemented a national rubella and CRS elimination program that included MR vaccination* for persons aged 15--39 years, in accordance with World Health Organization recommendations (3--5). Measles-containing vaccine was used in this campaign to maintain elimination of measles in Costa Rica. The last confirmed case of measles was reported in September 1999. During May 2001, the Ministry of Health and the Social Security System collaborated to vaccinate >95% of the adult population. The ministries of education and labor, worker's unions, religious leaders, community associations, student federations, university representatives, entrepreneurs, and local governments assisted with communication and social mobilization. During the 2 weeks preceding the vaccination campaign, news-papers, radio, and television stations informed the targeted adults about the importance of vaccination. During the campaign, vaccine was offered at health units and locations convenient for the target populations (e.g., malls, universities, and workplaces). In addition, mobile teams went house-to-house from sparsely populated areas to densely populated areas. Reports of doses administered were submitted daily by health units and periodically by selected workplace vaccination programs. These reports were used to estimate regional and national vaccination coverage by age group, sex, and canton (i.e., district) of residence. During the 4 weeks of the campaign, coverage of persons aged 15--45 years increased from 30% at the end of week 1, to 61%, 80%, and 98% for subsequent weeks, respectively. A total of 1,635,445 persons were vaccinated, representing 42% of the country's population (6,7). Vaccination coverage by age group was 111% (aged 15--19 years), 92% (aged 20--24 years), 93% (aged 25--29 years), 87% (aged 30--34 years), and 106% (aged 35--39 years). Coverage was >100% in the youngest and oldest targeted age groups because of the inclusion of vaccinated persons either younger or older than the targeted age. Vaccination coverage was at least 80% in all 81 cantons and >95% in 60 cantons. . Vaccine safety surveillance conducted by the Social Security System using a passive reporting system detected 981 events (rate: 60 per 100,000 vaccinated persons) possibly related to vaccination, including rash (26%), lymphadenopathy (16%), fever (15%), headache (10%), and arthralgias or arthritis (10%). Of >1.6 million doses administered, health-care workers reported five needlestick injuries at the time of vaccine preparation out . Women aged 15--40 years known to be pregnant (56,634 [7%]) at the time of the campaign were not vaccinated and will be vaccinated after delivery. Vaccinated persons were not eligible to donate blood for 1 month after vaccination, and blood donations decreased 52% in May compared with the previous 12 months. To maintain the blood supply, information about blood donation was distributed to persons not targeted for vaccination; persons aged >40 years had accounted for approximately 25% of blood donations before the campaign. During and immediately after the campaign, this group accounted for approximately 95% of donations. Blood donations returned to normal in July. Surveillance for measles and rubella in Costa Rica is integrated with the surveillance of febrile rash illnesses, including dengue fever and leptospirosis. In conjunction with the MR vaccination campaign, rubella and CRS surveillance protocols were updated, laboratory capabilities for isolating and identifying rubella virus were upgraded, and training programs were conducted for staff at the national epidemiologic surveillance unit. Reported by: Ministry of Health; Social Security System, Costa Rica. Div of Vaccines and Immunization, Pan American Health Organization, Washington, DC, Div of International Health, Epidemiology Program Office; Epidemiology and Surveillance Div; Global Measles Br, Global Immunization Div, National Immunization Program, CDC. Editorial Note:Adults are difficult to reach with mass vaccination campaigns possibly because vaccination usually is not considered part of adult health care. Aspects of the design and implementation of this vaccination campaign can serve as a model for other countries that want to eliminate CRS and rubella. Complete demographic information about the target population obtained through an up-to-date census or registry is useful in assuring adequate vaccine and staff, targeting the campaign to appropriate areas, and estimating coverage. Supplemental outreach activities can reach immigrants and persons residing in remote areas. Coordination between national authorities and local campaign organizers can avoid the occurrence of dangerously lowering blood reserves. Strategies include conducting a blood drive before the vaccination campaign, selecting a pool of donors to be vaccinated after the campaign, and offering incentives for blood donation among persons aged 40--60 years. Safety data should be gathered in a timely fashion to ensure the safety of vaccine and to address concerns about adverse events. The low number of needlestick injuries reflects the appropriate biosafety training given to vaccinators before the campaign. To maintain the goals of measles, rubella, and CRS elimination, Costa Rica will need to 1) achieve and maintain coverage >95% with measles- and rubella-containing vaccine at both scheduled vaccination opportunities or conduct periodic mass vaccination campaigns; 2) continue surveillance for measles, rubella, and CRS; and 3) adjust their vaccination strategy in response to new surveillance information. References
* MR vaccine manufactured by Serum Institute of India. Measured by the number of doses of rubella-containing vaccine administered to persons in the age group divided by the total population in that age group and multiplied by 100. Figure 1 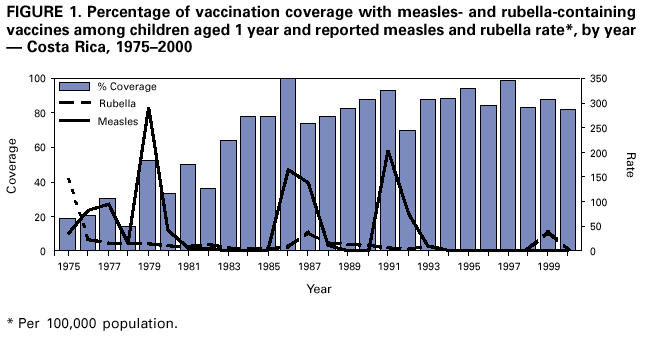 Return to top. Figure 2 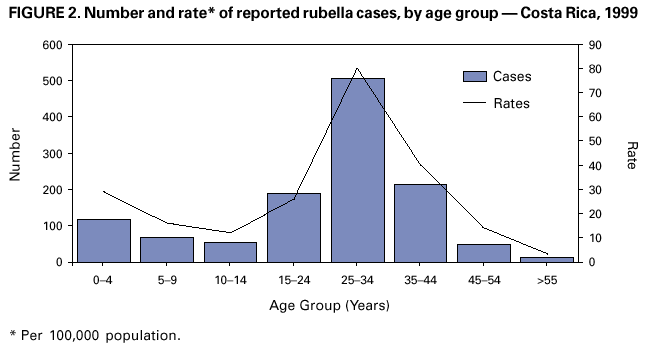 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/13/2001 |
|||||||||
This page last reviewed 11/13/2001
|