 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Human Anthrax Associated With an Epizootic Among Livestock --- North Dakota, 2000On August 28, 2000, the North Dakota Department of Health was notified by a local clinician of a patient with a cutaneous lesion suggestive of anthrax following exposure to an infected animal carcass. This report summarizes the investigation of this case, which was associated with an anthrax epizootic among livestock in North Dakota, and emphasizes the importance of increased vigilance for human cases of anthrax during and following outbreaks of anthrax among livestock. On August 19, 2000, a 67-year-old resident of eastern North Dakota participated in the disposal of five cows that had died of anthrax. On the day of disposal, he placed chains around the heads and hooves of the animals and moved them to a burial site. He reported having worn leather gloves throughout transportation and disposal. On August 23, he noticed a small bump on his left cheek at the angle of his jaw. On August 25, the lesion had enlarged and he sought medical attention. He denied fever, malaise, headache, pruritus, or difficulty swallowing. On examination, the lesion was indurated to approximately the size of a quarter and was surrounded by a purple colored ring. The patient was afebrile and did not appear ill. The physician reported a firm, nontender, superficial nodule with an overlying 0.5 cm black eschar. No drainage was noted and neither wound nor blood cultures was obtained. The patient was placed on ciprofloxacin 500 mg twice a day for presumed cutaneous anthrax. On follow-up examination on August 28, the eschar had enlarged to 1 cm. Following consultation with the North Dakota Department of Health and based on clinical suspicion of anthrax, the patient continued the course of ciprofloxacin for a total of 14 days. The lesion slowly improved over several weeks. Paired serum specimens were obtained on September 22 and October 5, 2000, and were tested at CDC; both had positive antibody titers by ELISA of 200 to protective antigen, confirming infection with Bacillus anthracis. This case was associated with an anthrax epizootic in North Dakota, during which 32 farms were quarantined for anthrax in 2000*, compared with an average of two farms per year during the preceding 40 years (Figure 1). The initial cases were detected in May 2000, when four animals were found dead on a farm; the deaths were later confirmed to be associated with anthrax. During the epizootic, which extended from July 6 through September 24, 2000, 157 animals died on 31 farms on which 62 persons were involved with animal care, vaccination, specimen processing, or carcass disposal. No other cases of symptomatic anthrax were identified in humans in North Dakota. Reported by: L Shireley, MPH, T Dwelle, MD, D Streitz, North Dakota Dept of Health; L Schuler, DVM, North Dakota Dept of Agriculture. Animal and Plant Health Inspection Svc, US Dept of Agriculture. Meningitis and Special Pathogens Br, Div of Bacterial and Mycotic Diseases, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:This report presents the first case of cutaneous anthrax in the United States since 1992. In the United States, the annual incidence of human anthrax declined from approximately 200 cases in the early 1900s to no human cases since 1992. Although most cases reported in the United States have been cutaneous, 18 cases of inhalational anthrax were reported during the 20th century, most recently in 1976 (1). No cases of gastrointestinal anthrax have been reported in the United States. Anthrax most commonly occurs in both wild and domestic mammals (e.g., cattle, sheep, goats, camels, antelopes, and other herbivores) (2). Humans develop anthrax infection following exposure to infected animals, tissue from infected animals, or by direct exposure to B. anthracis (3,4). Exposure to infected animal tissue can occur during postmortem examination, slaughter, or handling of infected meat or hides. Exposure also can occur during laboratory manipulation of infected blood, muscle, or other tissues. Human-to-human transmission of anthrax is rare. Anthrax can occur in three forms: cutaneous, gastrointestinal, and inhalational (2). Most cases (95% worldwide) are cutaneous. The incubation period for cutaneous anthrax ranges from 12 hours to 12 days (2--5). Cutaneous anthrax may begin with pruritus at the affected site, typically followed by a small, painless papule that progresses to a vesicle in 1--2 days. The lesion erodes, leaving a necrotic ulcer with a characteristic black center. Secondary vesicles are sometimes observed, lymphadenopathy may occur, and local edema may be extensive. Patients may have fever, malaise, and headache. The most common sites of cutaneous anthrax are the hands, forearms, and head. Of the 203 cases reported in the United States since 1955 in which the site of infection was known, 64 (27%) have been in the head and neck region (2). Presumably, the mechanism of inoculation in this case was the transfer of infective spores on the patient's gloves to broken skin on his face. Untreated, 20% of persons with cutaneous anthrax die, compared with <1% of those who receive antibiotic therapy (2,6). B. anthracis is sensitive in vitro to penicillin, tetracycline, chloramphenicol, and ciprofloxacin (7). In localized or uncomplicated cases of cutaneous anthrax, the recommended regimen is penicillin V, 500 mg taken orally every 6 hours for 5--7 days. For more severe cases of cutaneous anthrax, penicillin G, 4--6 million units every 6 hours intravenously for 7--10 days is recommended. Doxycycline, 100 mg twice a day for localized cases or intravenously for serious cases, also can be used (7--9). Veterinarians and agricultural workers should minimize direct contact with animals suspected to have died of anthrax. For confirmation by smear or culture, the carcass should not be opened, and a postmortem blood sample should be obtained aseptically by a veterinarian from an accessible peripheral vein (e.g., jugular vein). Specimens also can be obtained from hemorrhagic nasal, buccal, or anal exudate or from materials contaminated with the exudate. If possible, the carcass should be burned or buried where it is found. To minimize environmental contamination, burning is the preferred disposal method. Bedding and other materials found around the carcass (e.g., contaminated soil) also should be burned or buried, and all remaining animals should be promptly removed from the affected pasture. Farms where anthrax deaths among livestock are confirmed should be quarantined and all susceptible healthy livestock on the affected and neighboring premises vaccinated with the Sterne vaccine. Where anthrax is suspected or confirmed, use of long-acting antibiotics followed by vaccination may be effective in reducing livestock deaths. However, this regimen has not been systematically evaluated. Because this epizootic may continue in North Dakota and because anthrax cases among livestock occur each year, health-care providers should consider the possibility of anthrax when evaluating patients with characteristic skin lesions, particularly if the exposure history includes handling of animals with confirmed or suspected anthrax. Vigilance for human cases of anthrax should be heightened during anthrax epizootics. Veterinary health services should work closely with public and private health officials to ensure early detection and treatment of possible human anthrax cases resulting from exposure to animals during an epizootic. Any person who handles carcasses of animals that have died or are suspected to have died of anthrax should contact their health-care provider if they develop a skin lesion. Although veterinarians, agricultural workers, and laboratory workers might be at increased risk for B. anthracis infection during these epizootics, the risk is low and anthrax vaccination is not recommended (10). References
* A quarantined farm is one on which at least one case of culture-confirmed anthrax has occurred among livestock. All MMWR references are available on the Internet at <http://www.cdc.gov/mmwr>. Use the search function to find specific articles. Figure 1 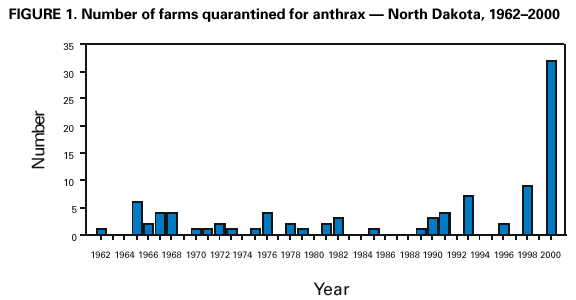 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 8/24/2001 |
|||||||||
This page last reviewed 8/24/2001
|