 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity --- United States and Worldwide, 2000--01 Season, and Composition of the 2001--02 Influenza VaccineThe 2000--01 influenza season was mild in the United States and was the first season since 1995--96 that was not predominated by A (H3N2) viruses. Influenza A (H1N1) viruses predominated in the United States. In some regions, however, influenza B viruses were reported more frequently than influenza A viruses. Worldwide, influenza A (H1N1) and B viruses also predominated. This report summarizes U.S.* and worldwide influenza activity during the 2000--01 influenza season and describes the composition of the 2001--02 influenza vaccine. United StatesInfluenza activity increased in mid-December and peaked from mid-January through early February. Influenza A (H1N1) viruses predominated; however, the number of influenza type B viruses increased as the season progressed. Influenza B viruses were more frequently identified than influenza A viruses from the week ending February 10 through the week ending May 19 and were the predominant virus type identified in three of the nine surveillance regions. World Health Organization and National Respiratory and Enteric Virus Surveillance System collaborating laboratories in the United States tested 88,598 respiratory specimens for influenza during October 1, 2000--May 19, 2001; 9962 (11%) were positive (Figure 1). Of these, 5337 (54%) were positive for influenza type A and 4625 (46%) were positive for influenza type B. Of the 2127 subtyped influenza A viruses, 2061 (97%) were type A (H1N1) and 66 (3%) were A (H3N2). Influenza type B viruses were isolated more frequently than type A viruses from the week ending February 10 through the week ending May 19. Influenza type A viruses predominated in the East North Central, South Atlantic, West North Central, and West South Central regions; influenza B viruses predominated in the mid-Atlantic, Mountain, and Pacific regions. The East South Central and New England regions reported approximately equal numbers of influenza A and B viruses. The proportion of specimens testing positive for influenza first increased to >10% during the week ending December 23, 2000, peaked at 24% during the week ending January 27, 2001, and declined to <10% during the week ending March 10. The peak percentage of specimens testing positive for influenza during the 2000--01 season was lower than that seen during the previous three seasons when the peak ranged from 28% to 32%. CDC antigenically characterized 678 influenza viruses received from U.S. laboratories since October 1; 335 (95%) of the 354 influenza A (H1N1) viruses were similar to A/New Caledonia/20/99, the H1N1 component of the 2000--01 influenza vaccine, and 19 (5%) were similar to A/Bayern/07/95. Although A/Bayern-like viruses are distinct from the A/New Caledonia-like viruses, the A/New Caledonia/20/99 vaccine strain produces high titers of antibody that cross-react with A/Bayern/07/95-like viruses. Of the 23 influenza A (H3N2) viruses that were characterized, all were similar to the vaccine strain A/Panama/2007/99. Of the 301 influenza B viruses that were characterized, 33 (11%) were similar to the vaccine strain, B/Beijing/184/93, and 268 (89%) were most closely related to the B/Sichuan/379/99 reference strain. U.S. influenza sentinel physicians reported that the percentage of patient visits for influenza-like illness (ILI) exceeded baseline levels (0--3%) for 4 consecutive weeks from the week ending January 20 through the week ending February 10. During each of the 4 weeks, 4% of patient visits were for ILI. During the previous three influenza seasons, the peak percentage of patient visits for ILI ranged from 5% to 7%. On the basis of data from state and territorial epidemiologists' reports, influenza activity peaked during the weeks ending February 3 and February 10, when 38 states reported regional or widespread influenza activity§. State and territorial epidemiologists reported regional influenza activity during consecutive weeks from the week ending November 18 through the week ending March 31. Widespread activity was reported by one or more states during consecutive weeks for the week ending January 6 through the week ending March 10. The peak number of states reporting regional or widespread activity during the previous 3 years ranged from 43 to 46. As reported through the 122 Cities Mortality Reporting System, the percentage of deaths in the United States associated with pneumonia and influenza (P&I) did not exceed the epidemic threshold¶ during the 2000--01 influenza season. During the previous three seasons, the percentage of deaths attributed to P&I was above the epidemic threshold for 10 consecutive weeks each season. WorldwideDuring October 2000--April 2001, influenza A (H1N1) and influenza B viruses circulated widely in Africa, the Americas, Asia, and Europe and influenza A (H3N2) viruses were reported sporadically. Influenza A (H1N1) viruses predominated in most European countries (Albania, Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Italy, Latvia, the Netherlands, Norway, Romania, Russia, Slovakia, Spain, Switzerland, Ukraine, and Yugoslavia). Influenza A (H1N1) viruses also were reported from Asia (China, Hong Kong/China, Iran, Israel, Japan, Republic of Korea, Nepal, Singapore, and Thailand), Africa (Mauritius and Morocco), the Americas (Canada, Jamaica, Mexico, Panama, and Peru), other European countries (Iceland, Ireland, Portugal, Sweden, and the United Kingdom), and Oceania (Australia). Influenza type B viruses were identified more frequently than influenza A viruses in Canada, China, Egypt, Iceland, Ireland, Hong Kong/China, Portugal, Sweden, and the United Kingdom. Influenza type B viruses also were reported in Argentina, Australia, Brazil, Chile, India, Israel, Japan, Republic of Korea, Mauritius, Paraguay, Peru, Singapore, Taiwan, Thailand, and throughout Europe. Influenza A (H3N2) viruses were identified in Argentina, Australia, Brazil, Bulgaria, Canada, Chile, China, Czech Republic, Denmark, France, Germany, Hong Kong/China, Ireland, Italy, Japan, Republic of Korea, Peru, Portugal, Russia, Singapore, South Africa, Spain, Switzerland, Taiwan, and Thailand. Unsubtyped influenza A viruses were reported from Belarus, Chile, Colombia, Malaysia, Nepal, Paraguay, and Slovenia. Composition of the 2001--02 Influenza VaccineThe Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee (VRBPAC) recommended that the 2001--02 trivalent influenza vaccine for the United States contain A/New Caledonia/20/99-like (H1N1), A/Moscow/10/99-like (H3N2), and B/Sichuan/379/99-like viruses. This recommendation was based on antigenic analyses of recently isolated influenza viruses, epidemiologic data, and postvaccination serologic studies in humans. Most influenza A (H1N1) viruses isolated worldwide were similar to A/New Caledonia/20/99 (H1N1). Both A/New Caledonia/20/99 and A/Bayern/07/95-like (H1N1) viruses circulated in the United States. Although these viruses antigenically are distinct, antibodies produced against A/New Caledonia/20/99 react at equivalent levels with A/Bayern/07/95-like viruses (2); therefore, A/New Caledonia/20/99 was retained in the 2001--02 influenza vaccine. Most influenza A (H3N2) viruses isolated during the 2000--01 season were similar to A/Panama/2007/99 and A/Moscow/10/99-like (H3N2) viruses. Antibodies produced following vaccination with the 2000--01 vaccine containing the A/Panama/2007/99 (H3N2) virus reacted equally well with recent influenza A (H3N2) viruses and the vaccine strain (2); therefore, VRBPAC recommended that an influenza A/Moscow/10/99-like (H3N2) virus be retained in the 2001--02 vaccine. Because of its growth properties, U.S. vaccine manufacturers will use the antigenically equivalent virus, A/Panama/2007/99. Most influenza B isolates were related more closely to the antigenic drift variant B/Sichuan/379/99 than the 2000--01 B/Beijing/184/93-like vaccine strain, B/Yamanashi/166/98. Antibodies produced against the B/Yamanashi/166/98 vaccine strain cross-reacted with B/Sichuan/379/99-like viruses; however, antibodies were lower in titer and frequency against B/Sichuan/379/99-like viruses than B/Yamanashi/166/98-like viruses (2). Therefore, VRBPAC recommended that the influenza B component be updated for the 2001--02 vaccine to an influenza B/Sichuan/379/99-like virus. For the B/Sichuan/379/99-like virus, U.S. manufacturers will use one of the antigenically equivalent viruses B/Johannesburg/05/99, B/Victoria/504/2000, or B/Guangdong/120/2000. Reported by: World Health Organization National Influenza Centers, Communicable Diseases, Surveillance and Response, World Health Organization, Geneva, Switzerland. A Hay, PhD, WHO Collaborating Center for Reference and Research on Influenza, National Institute for Medical Research, London, England. I Gust, MD, A Hampson, WHO Collaborating Center for Reference and Research on Influenza, Parkville, Australia. M Tashiro, MD, WHO Collaborating Center for Reference and Research on Influenza, National Institute of Infectious Diseases, Tokyo, Japan. Participating state and territorial epidemiologists and state public health laboratory directors. WHO collaborating laboratories. National Respiratory and Enteric Virus Surveillance System laboratories. Sentinel Physicians Influenza Surveillance System. Surveillance Systems Br, Div of Public Health Surveillance and Informatics, Epidemiology Program Office; WHO Collaborating Center for Reference and Research on Influenza, Influenza Br and Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial Note:Influenza A (H1N1) and B viruses co-circulated in the United States and worldwide during the 2000--01 influenza season. Influenza A (H3N2) viruses were isolated sporadically and no country reported widespread activity as a result of influenza A (H3N2) viruses. Seasons in which influenza A (H1N1) and/or influenza B viruses predominate typically have been less severe than seasons in which influenza A (H3N2) viruses circulate widely (3). The level of influenza activity reported this season was consistent with a number of other A (H1N1) and B predominant years. Although influenza epidemics typically peak during December--March in the temperate regions of the Northern Hemisphere, sporadic cases and outbreaks can occur during the summer (4--6). The influenza season typically peaks during May--August in temperate regions of the Southern Hemisphere, and epidemics can occur at any time of the year in the tropics. U.S. physicians should consider influenza when diagnosing a febrile respiratory illness during the summer, particularly among persons who have traveled recently in the tropics, Southern Hemisphere, or with large international groups. Persons at increased risk for influenza-related complications who were not vaccinated during the preceding fall or winter should consider receiving influenza vaccine, if available, before traveling to the tropics, Southern Hemisphere, or with large international groups. Persons at increased risk for influenza-related complications who have received the previous season's vaccine during the summer should be vaccinated with the current influenza vaccine during the following fall or winter. References
* The four components of the influenza surveillance system have been described (1). Information reported as of June 5, 2001. Temperature >100.0 F (>37.8 C) and either cough or sore throat in the absence of a known cause. § Levels of activity are 1) no activity; 2) sporadic---sporadically occurring ILI or cultureconfirmed influenza with no outbreaks detected; 3) regional---outbreaks of ILI or cultureconfirmed influenza in counties with a combined population of <50% of the state's population; and 4)widespread---outbreaks of ILI or cultureconfirmed influenza in counties with a combined population of >50% of the state's population. ¶ Before the 1999--2000 season, the case definition for P&I deaths was modified. CDC analysis estimated that the revised case definition resulted in an average increase in baseline P&I mortality estimates of 0.8% for 1999--2000. Thus, the 122 cities P&I mortality baseline and epidemic threshold for the 2000--01 season have been adjusted upward. The epidemic threshold is 1.645 standard deviations above the seasonal baseline. The expected seasonal baseline is projected using a robust regression procedure in which a periodic regression model is applied to observed percentages of deaths from P&I since 1983.
Figure 1 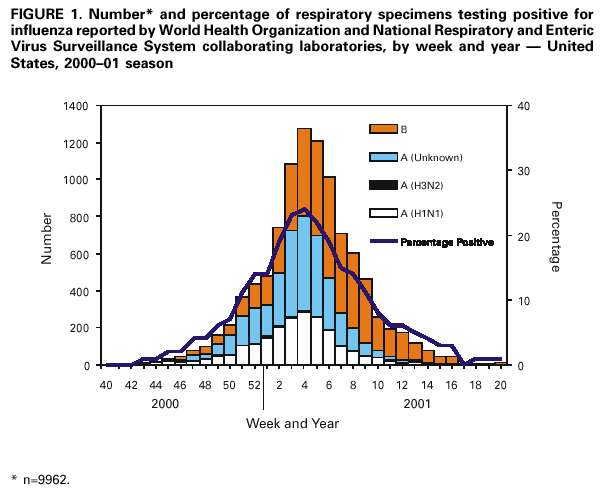 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 6/8/2001 |
|||||||||
This page last reviewed 6/8/2001
|