 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Hepatitis C Virus Infection Among Firefighters, Emergency Medical Technicians, and Paramedics --- Selected Locations, United States, 1991--2000First responders (e.g., firefighters, emergency medical technicians [EMTs], and paramedics) are at risk for occupational exposure to bloodborne pathogens. Recently, CDC has received inquiries from state and local health departments and occupational health services about the prevalence of hepatitis C virus (HCV) infection among first responders and the need for routine HCV testing among these workers. This report summarizes the findings of five studies of HCV infection among first responders. Although some of these workers may need HCV testing under certain circumstances, this report indicates that first responders are not at greater risk than the general population for HCV infection; therefore, routine HCV testing is not warranted. First responders should continue to follow standard precautions to reduce workplace exposure to bloodborne pathogens. Philadelphia, PennsylvaniaDuring November--December 1999, Home Access Health Corporation (Hoffman Estates, Illinois)* offered specimen collection kits (Hepatitis C Check) to 4400 active and retired members of the Philadelphia firefighters union. Respondents telephoned a toll-free number to receive their test results and to answer questions anonymously about nonoccupational risk factors for HCV infection. According to Home Access®, serum was tested for antibody to HCV (anti-HCV) with an enzyme immunoassay (EIA 3.0; Ortho Diagnostic Systems, Inc., Raritan, New Jersey); repeatedly reactive samples were tested with a supplemental recombinant immunoblot assay (RIBA 3.0, Chiron Corporation, Emeryville, California). In February 2000, Home Access reported that of 2146 respondents, 97 (4.5%) screened positive for anti-HCV. The company indicated that this prevalence was 2.5 times higher than the national average of 1.8% (Home Access Health Corporation, personal communication, 2000). In June 2000, CDC re-analyzed serologic and questionnaire data and found that of 2136 participants, 64 (3.0%) tested anti-HCV--positive (Table 1). The highest prevalence (4.9%) was among men aged 40--49 years (Figure 1). Risk factors associated with HCV infection were a history of blood transfusion before 1992 (age-adjusted prevalence ratio [PR]=2.2; 95% confidence interval [CI]=1.2--4.0) and illicit drug use (age-adjusted PR=4.0; 95% CI=2.2--7.1). On the basis of CDC's analysis, the 4.5% prevalence previously reported by Home Access was obtained by classifying as positive samples that tested EIA repeatedly reactive but indeterminate by RIBA, and those that tested EIA repeatedly reactive or EIA initially reactive for which no further testing was done (Table 2). Atlanta, GeorgiaIn 1991, CDC conducted a voluntary, anonymous survey among metropolitan Atlanta uniformed fire department personnel to assess occupational and nonoccupational risk factors for hepatitis B virus (HBV) infection (1). In May 2000, stored serum samples were tested at CDC for anti-HCV using EIA 3.0; repeatedly reactive samples were tested by RIBA 3.0. Of the 437 firefighters tested, nine (2.1%) were anti-HCV--positive (Table 1); the highest prevalence (4.0%) was among men aged 35--39 years. HCV infection was not associated with duration of employment as a firefighter, occupational exposures to blood, history of blood transfusion, or illicit drug use; however, HCV infection was associated with a history of a sexually transmitted disease (PR=7.4; 95% CI=1.6--35.3). ConnecticutIn 1992, Connecticut Department of Public Health and Addiction Services collected serum samples and demographic data on a voluntary basis from first responders in various regions in Connecticut for a study on the immune response to hepatitis B vaccine (2). In June 2000, stored serum samples from the 1992 study were tested anonymously at CDC for anti-HCV by EIA 3.0 and RIBA 3.0. Among 382 volunteer and professional firefighters and EMTs for whom serum samples were available, five (1.3%) tested anti-HCV--positive (Table 1); prevalence was highest (2.6%) among men aged 40--49 years. Miami-Dade County, FloridaDuring March--April 2000, Hep-C ALERT, a patient advocacy organization, collaborating with University of Pittsburgh researchers, confidentially obtained serum samples and information on occupational risk factors from Miami-Dade County municipal fire department personnel. Serum samples were tested at a commercial laboratory for anti-HCV with EIA 3.0; repeatedly reactive samples were tested for HCV RNA by transcription mediated amplification (TMA) (Bayer Corporation, Tarrytown, New York). Of the 1314 participants, 35 (2.7%) were anti-HCV--positive on the basis of EIA testing alone, and 20 (1.5%) were confirmed positive for HCV RNA (Table 1). Prevalence of anti-HCV was highest (3.7%) among men aged >50 years. Increased risk for HCV infection was not associated with occupational exposures to blood, type of job (firefighter, EMT, or paramedic), or duration of employment as a first responder. Pittsburgh, PennsylvaniaDuring January--March 2000, University of Pittsburgh researchers collected serum samples and information on occupational exposures from paramedics working in Pittsburgh. Samples were tested for anti-HCV by EIA 2.0 (Abbott Laboratories, Abbott Park, Illinois) without supplemental or confirmatory testing. Five (3.2%) of 154 respondents tested anti-HCV--positive (Table 1); highest prevalence (5.2%) was among men aged 40--49 years. Anti-HCV positivity was not associated with occupational exposures to blood. Reported by: AJ Roome, PhD, HIV/AIDS Surveillance Program, JL Hadler, MD, State Epidemiologist, Connecticut Dept of Public Health. AL Thomas, B Migicovsky, MD, Hep-C ALERT, Miami. MW Dailey, MD, R Roth, MD, Dept of Emergency Medicine, Univ of Pittsburgh; M Boraz, PhD, Graduate School of Public Health, Univ of Pittsburgh; B Kuszajewksi, D Berkowitz, MPH, Bur of Emergency Medical Svcs, City of Pittsburgh, Pennsylvania. Hepatitis Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; and an EIS Officer, CDC. Editorial Note:Data from the Third National Health and Nutrition Examination Survey (NHANES III), conducted during 1988--1994, indicated that 3.9 million (1.8%) persons living in the United States have been infected with HCV (3). Estimates indicate that three risk factors accounted for most infections: illicit drug use (60%), high-risk sexual behavior (15%), and blood transfusion (7%) (CDC, unpublished data, 1996; 3,4). Health-care workers and first responders exposed to blood in the workplace are at risk for infection by bloodborne pathogens. However, their risk for acquiring HCV infection is low because HCV is not transmitted efficiently through occupational exposure (4--6). After an unintentional needlestick from an HCV-positive source, the average risk for HCV infection is 1.8% (range: 0--7%); transmission rarely occurs from mucous membrane exposures to blood, and no transmission has been documented from intact or nonintact skin exposures to blood (4). Among first responders, HCV infection was associated primarily with nonoccupational factors, a finding similar to HBV (1), a bloodborne virus that is transmitted at a rate 10 times higher than HCV (7). The initial interpretation of the results from the Philadelphia study was incorrect because 20.6% of the serum samples classified as positive were of insufficient volume to complete testing as required by the Food and Drug Administration (FDA). Manufacturer's instructions for performing EIA for anti-HCV require initially reactive samples to be repeated in duplicate; only samples that are repeatedly reactive are considered EIA-positive. For Hepatitis C Check, FDA-approved conditions for reporting a positive anti-HCV result require a repeatedly reactive EIA and a positive supplemental test. Samples with insufficient volume for supplemental testing are to be reported as "results not available --- insufficient blood." In populations with an HCV-infection prevalence of 0--10%, 20%--50% of EIA repeatedly reactive results may be false positives (4,8). HCV prevalence reported in studies in subpopulations should be compared with appropriate referent groups from the general population. In NHANES III, conducted during 1988--1994, overall prevalence of HCV infection among persons of both sexes aged >5 years was 1.8% but was substantially higher (4.9%) among men aged 30--49 years (3), the group that represents most of the first responders in the five studies. Because this group has aged almost 10 years since NHANES III was conducted, men currently aged 40--59 years would have the highest expected prevalence of infection (Figure 1). Because of several limitations, the five studies could not exclude the possibility that some first responders had acquired HCV infection from job-related exposures. First, the small sample size and limited information on both occupational (percutaneous, mucosal, or skin exposures to blood) and nonoccupational risk factors may have affected the evaluation of potential sources for infection. Second, the findings do not necessarily represent all first responders in the selected locations or the United States. Third, if first responders are less likely to have nonoccupational risk factors for HCV infection than the general population, then the expected prevalence in these workers might be lower. Routine HCV testing is not recommended for populations with a low prevalence of HCV infection, including first responders, unless they have a history indicating an increased risk for infection (e.g., transfusion before July 1992 or injecting-drug use) (4). Testing is recommended in first responders for postexposure management after a percutaneous or permucosal exposure to HCV-positive blood(4), and testing could be considered for these types of exposures when the HCV status of the source is unknown (9). To reduce workplace exposure to bloodborne pathogens, standard precautions continue to apply; first responders should be educated about transmission of bloodborne pathogens, trained in proper safety measures, and provided with appropriate protective equipment (10). First responders also should be vaccinated against HBV, and informed of protocols if percutaneous or permucosal exposures to blood occur (4,10). References
* Use of trade names and commercial sources is for identification only and does not constitute endorsement by CDC or the U.S. Department of Health and Human Services. Bloodborne pathogens, 29 CFR sect. 1910.1030 (1999). Table 1 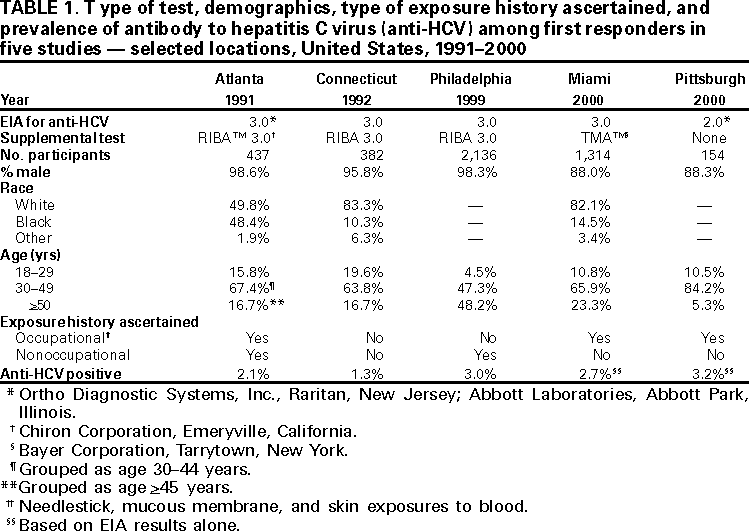 Return to top. Figure 1 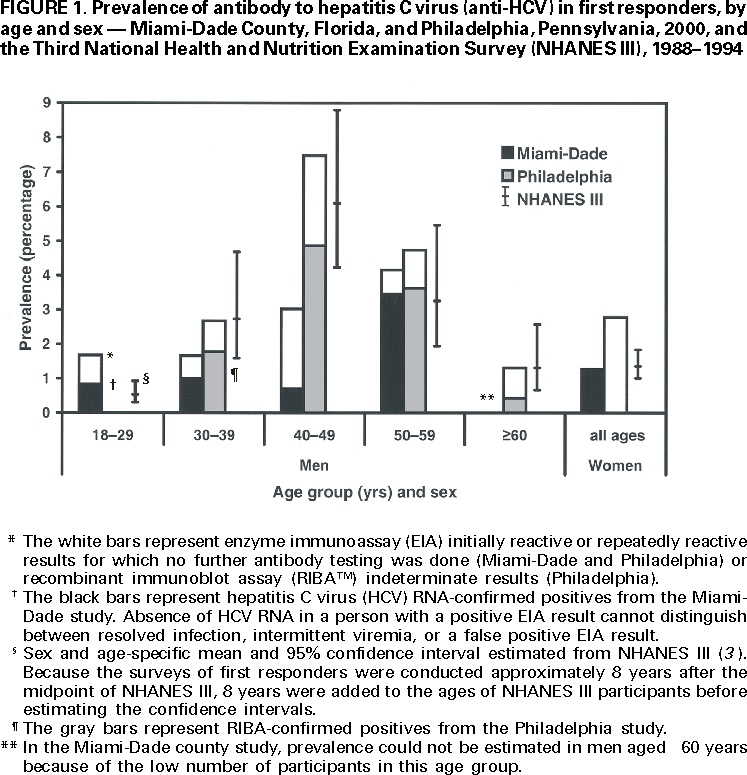 Return to top. Table 2 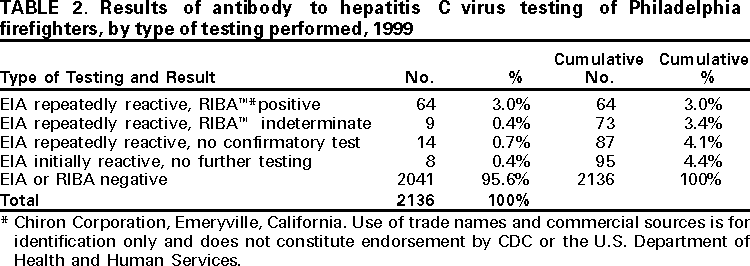 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/27/2000 |
|||||||||
This page last reviewed 5/2/01
|