 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Adverse Events Associated with Anthrax Vaccination --- U.S. Department of Defense, 1998--2000Concerns about the potential use of anthrax as a biologic weapon prompted the U.S. Department of Defense (DoD) to announce on December 15, 1997, anthrax vaccination of all U.S. military personnel. This effort is coordinated by the Anthrax Vaccine Immunization Program (AVIP). AVIP plans a phased vaccination process to achieve total force protection against anthrax by 2004. The current phase of implementation includes vaccination of all service members and mission-essential DoD civilian employees assigned or deployed to high-threat areas. On the basis of program monitoring, as of April 12, 2000, 425,976 service members had received 1,620,793 doses of anthrax vaccine adsorbed (AVA) (Bioport, Inc.,* Lansing, Michigan). Some service members have cited concerns about vaccine safety and efficacy in their decision to refuse vaccination, despite the possibility of administrative or disciplinary actions. To assess anthrax vaccination safety, DoD has conducted surveys of vaccinated personnel. This report describes three completed or ongoing surveys (1). The findings indicate that rates of local reactions were higher in women than men and that no patterns of unexpected local or systemic adverse events have been identified. Survey of Self-Reported Reactions to AVA, U.S. Forces, KoreaAt one of the largest vaccination sites for United States Forces, Korea, a mandatory, self-administered prevaccination questionnaire was used to obtain data on health status (including pregnancy, if applicable), medication use, and reactions to the previous dose of AVA. The questionnaire was designed to record service members' concerns about AVA and their reports of adverse events (i.e., a medical condition following vaccination that could be related to the vaccine) to promote risk communication between health-care providers and service members. Data from 6879 questionnaires completed during September--October 1998 were reviewed. Approximately 37% (2531 of 6879) of respondents were service members receiving their first dose; records were analyzed for 4348 (63%) service members who already had received and could comment on their first (2427) or second (1921) vaccine doses. Female service members reported higher rates of reactions to the previous dose of vaccine, regardless of the time period after vaccination (Figure 1). For both men and women, most reported that events were localized, minor, self-limited, and did not lead to impaired work performance, lost work time beyond that required to seek care, and/or a clinic visit or hospitalization. After the first or second dose, 82 (1.9%) of 4348 reported that their work performance had been limited to some extent or that they were placed on limited duty, 13 (0.3%) reported <1 day lost from work, 21 (0.5%) consulted a clinic for evaluation, and one (0.02%) required hospitalization for an injection site reaction. Tripler Army Medical Center Survey of AVA SafetyTripler Army Medical Center, Honolulu, Hawaii, assessed the frequency and nature of AVA adverse events in a cohort of 603 U.S. military health-care workers in the Korea Medical Augmentee Program. These personnel began receiving anthrax vaccination during September 1998. A self-administered questionnaire was used to collect data prospectively for systemic reactions. Data on local reactions were collected retrospectively for the first three doses and prospectively for the remaining doses. Persons responded to questions on symptoms, signs, time taken off from duty, hospitalizations, and medical visits. Medical records were reviewed and information was obtained from health-care providers about participants who sought medical care, missed one or more work shifts, or had any reaction that might exempt them from further vaccination. Data collection up to the fourth AVA dose of the six-dose initial series was complete for 479 (79.4%) of 603 persons. Of the remaining 124 (20.6%), 11 were not vaccinated because of pregnancy, four were exempted from the survey for medical reasons, and the rest were lost to follow-up primarily because of reassignment. After the first anthrax dose, 47 (7.9%) of 595 reported seeking medical advice and/or taking time off work for a complaint (e.g., muscle or joint aches, headache, or fatigue); after the second dose, 30 (5.1%) of 585; after the third dose, 16 (3.0%) of 536; and after the fourth dose, 17 (3.1%) of 536. Vaccine Adverse Events Reporting System (VAERS)DoD uses the CDC and Food and Drug Administration (FDA) Form VAERS-1 to report events potentially related to any vaccination to VAERS and to each military service's disease reporting system. VAERS reports related to anthrax vaccinations are consolidated for AVIP by the Defense Medical Surveillance System. As of April 7, 2000, 428 VAERS-1 reports had been received through DoD. Of these, 311 (72.7%) concerned systemic reactions, 78 (18.2%) were reports on mild or moderate local reactions, and 39 (9.1%) were for large or complicated local reactions. Thirty-six (8.4%) reactions met the DoD mandatory reporting criteria (i.e., hospitalization and/or time off duty >24 hours). None were related to suspected vaccine lot contamination. A panel of civilian scientific and medical experts established by the U.S. Department of Health and Human Services at DoD's request reviewed all VAERS-1 reports, including those reported directly to FDA or CDC. As of March 21, 2000, the panel has not identified any unexpected patterns of adverse events among 674 reports reviewed. Reported by: PA Sato, MD, M Ryan, MD, GC Gray, MD, Naval Health Research Center, San Diego, California. KJ Hoffman, MD, CN Costello, MD, United States Forces, Korea. GM Wassermann, MD, Tripler Army Medical Center, Honolulu, Hawaii. MV Rubertone, MD, SA Stanek, DO, US Army Center for Health Promotion and Preventive Medicine, Aberdeen Proving Grounds, Maryland. JD Grabenstein, PhD, Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, Falls Church, Virginia. JR Riddle, DVM, D Trump, MD, Office of the Assistant Secretary of Defense, US Dept of Defense, Washington, DC. Editorial Note:Anthrax is considered a biologic weapons threat because of its stability in spore form, its ease of culture, the absence of natural immunity in industrialized nations, and severity of infection in its gastrointestinal and inhalational forms. If untreated, the case-fatality rate of inhaled anthrax exceeds 80% (2,3). At least seven nations are suspected to have actively pursued biologic weapons programs (3,4). Anthrax also has been used at least once by terrorist groups (3,4). U.S. service members deployed to future military confrontations may be at risk for attack by biologic warfare agents. The DoD, through the AVIP, seeks to reduce these threats. Human anthrax vaccine was licensed by FDA in 1970 as a six-dose primary series with annual boosters. It is an aluminum hydroxide-adsorbed, cell-free, noninfectious vaccine prepared from a noncapsulating, nonproteolytic anthrax strain. Licensing was based on safety data, the results of a controlled efficacy trial, and observational data documenting substantial protection against anthrax (5,6). Studies in nonhuman primates also have documented protection (7). The safety and efficacy of this vaccine was affirmed by an independent advisory panel in 1985 (5). This mandatory vaccination program has posed substantial challenges to DoD. Some service members are reluctant to be vaccinated because of concern about adverse events. These concerns may be heightened by the number of doses required and by allegations linking vaccination with illnesses in Gulf War veterans. Conversely, some service members may not report adverse events after vaccination because of concerns that they will not be able to complete the vaccination series, thereby limiting career advancement options. The findings in this report provide information on rates of local and systemic adverse events occurring after anthrax vaccination was delivered as part of a routine program rather than in clinical trials. The findings suggest that rates of local reactions were higher in women than men and that no patterns of unexpected local or systemic adverse events have been identified. Reasons for the higher rates in women are unknown. The studies reported here are subject to several methodologic limitations, including sample size, the power to detect rare adverse events, loss to follow-up, and exemption of vaccine recipients with previous adverse events. For example, in the U.S. Forces, Korea, study, any service members medically deferred after a previous AVA dose would have been missed by the survey; therefore, adverse events may have been underreported. In the Tripler survey, data were collected retrospectively for the first three doses and then prospectively, potentially resulting in recall or observational bias. In addition, in the Tripler survey, the absence of an unvaccinated control group limited the ability to assess an association of adverse events with anthrax vaccination. The studies were not designed to detect or quantify chronic or long-term adverse events. Ongoing activities at DoD, CDC, and FDA are targeted toward improving methods to communicate the benefits and risks for vaccination, enhancing surveillance for vaccine adverse events, and continuing to monitor the safety of the program. These interventions may be useful to enhance AVIP. Risk-communication programs, such as the one in U.S. Forces, Korea, encourage a positive and supportive patient-provider relationship. Surveillance through the VAERS system to detect signals of potential adverse events followed by epidemiologic investigations to evaluate these signals remains important. Potential methodologies for monitoring safety include comparing vaccinated and unvaccinated groups or comparing groups shortly after vaccination with groups whose vaccinations were more distal. Pilot studies have evaluated intramuscular vaccine administration to reduce rates of local adverse events. Additional studies are planned to expand these data and to determine whether the number of doses required in the primary vaccination series can be reduced while maintaining immunogenicity and protection. AVIP maintains a World-Wide Web site (http://www.anthrax.osd.mil) with information on the program and electronic mail access to AVIP staff. A toll-free information line for inquiries from health-care providers, service members, and the public also is available (telephone [877] 438-8222). References
* Use of trade names and commercial sources is for identification only and does not imply endorsement by CDC or the U.S. Department of Health and Human Services. References to sites of non-CDC organizations on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. Figure 1 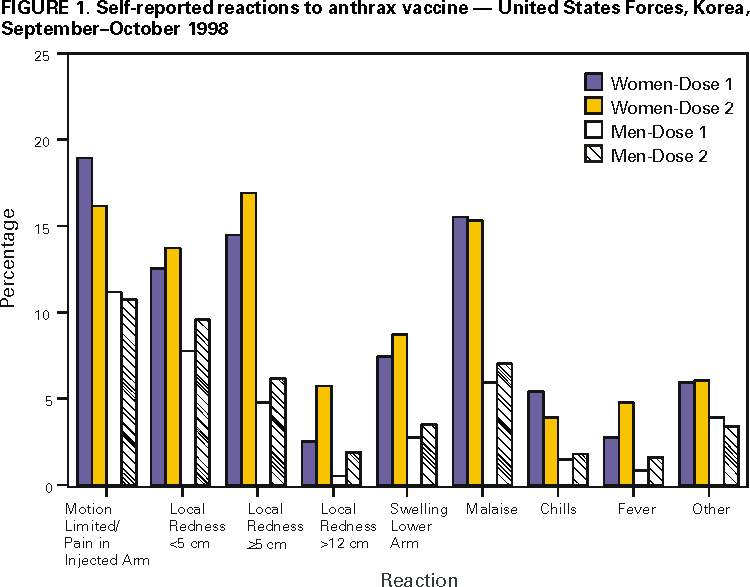 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/27/2000 |
|||||||||
This page last reviewed 5/2/01
|