 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Dengue Outbreak Associated with Multiple Serotypes -- Puerto Rico, 1998Dengue is an acute viral disease caused by any of the four dengue virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). The principal mosquito vector is Aedes aegypti, which has a worldwide distribution in tropical and many subtropical areas. All four virus serotypes produce a similar illness characterized by fever, headache, myalgias, arthralgias, rash, nausea and vomiting and induce life-long immunity that is specific to the infecting serotype (1). A small proportion of infected persons may develop the severe form of disease, dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS), but with early diagnosis and proper supportive management, fatality rates may be less than 1%. This report summarizes an epidemic of dengue in Puerto Rico in 1998 associated with multiple dengue serotypes. The laboratory-based dengue surveillance system of the Puerto Rico Department of Health (PRDH) and CDC receives diagnostic specimens and clinical information on a standardized form from government clinics, public and private hospitals, laboratories, and private physicians throughout Puerto Rico. In addition, infection-control nurses at all 56 general acute-care hospitals are asked to provide a voluntary report of demographic and clinical information on patients hospitalized with a diagnosis of suspected dengue fever. Cases are assigned to the date of onset of symptoms. From January 1 through August 29, 1998, 9803 cases of suspected dengue (i.e., disease in persons for whom a diagnostic serum sample was submitted) were reported. A total of 4677 (47.7%) were diagnosed as dengue by virologic or serologic testing, 526 (5.4%) were negative, and 4600 (46.9%) were indeterminate (i.e., testing was not complete or acute-phase serum was negative and no convalescent-phase sample was submitted). At the peak of the epidemic, the number of cases reported was approximately six times that expected for the time of year, based on a 5-year average (Figure_1). Of the 78 municipalities on the island, 67 (86%) had a statistically significant increase in reported cases, and 68 (87%) had a laboratory-diagnosed case (detection of antidengue IgM). Of 564 virus isolates, DEN-4 (45%) and DEN-1 (40%) predominated, followed by DEN-2 (12%), and DEN-3 (3%). In both reported and laboratory-positive cases, the male: female ratio was 1:1, and ages ranged from 0 to 98 years (median: 23 years). The islandwide attack rate was 2.8 per 1000 population based on the 1990 census. Age group-specific attack rates of reported disease were highest for persons aged 10-19 years (3.7; n=2494), and decreased with increasing age (1.7 among persons aged 65-98 years). A total of 4190 (42.7%) case-patients were hospitalized, and case report forms of 2888 (29.5%) noted some hemorrhagic manifestation. A DHF diagnosis requires documentation of excessive vascular permeability (hemoconcentration greater than or equal to 20%, hypoalbuminemia, or pleural or abdominal effusions), fever, platelet count less than or equal to 100,000/mm3, and any hemorrhagic manifestation (2). In 88 reports (30 {34%} laboratory-positive), sufficient information was included in the report to allow classifying the patient as having DHF. The highest rate of DHF (5.6 per 100,000 population) occurred in persons aged 55-59 years. Five persons (three males) with a positive laboratory diagnosis of dengue were reported to have died; decedents ranged in age from 8 months to 90 years (median: 19 years). However, only the infant had an illness meeting the case definition for DHF. From January through August 1998, 17 cases of DEN-3 infection were documented in Puerto Rico: 12 occurred among males. Case-patients ranged in age from 6 to 83 years (median: 16 years); 12 were hospitalized. Sixteen cases occurred among persons residing in municipalities in the northern half of the island, with a distance of approximately 70 miles (110 km) between the most distant points. These patients denied any travel outside Puerto Rico for at least 5 weeks before onset of illness. An additional DEN-3 case acquired outside Puerto Rico was identified in July. Analysis of the nucleotide sequence of the entire glycoprotein gene of the first two DEN-3 viruses isolated in Puerto Rico in 1998 showed that they were genetically distinct from the DEN-3 that occurred in the Americas from 1963 to 1977 and belong to the genotype (group III) that caused DHF epidemics in Sri Lanka and India in 1989 and 1990 (3). This same genotype, first detected in Central America (Nicaragua and Panama) in late 1994, also produced epidemics of dengue and DHF throughout the region (4-6). As part of the investigation of the initial DEN-3 cases, a survey of 45 premises around the second DEN-3 patient's residence indicated that 27 had one or more containers positive for Ae. aegypti larvae or pupae (Premise Index=60.0%), and 60 containers were positive (Breteau Index=133). Community residents had a high level of knowledge about Ae. aegypti larval habitats and of dengue as an illness and how it is transmitted. In February 1998, as part of the response to each of the first two DEN-3 isolates, PRDH alerted the public through the news media to immediately empty, eliminate, or seal all containers that hold water and to do this each week. Active disease surveillance was intensified, and sentinel locations were established in hospitals in the north and south of the island for dengue diagnosis among children with undifferentiated febrile illnesses. Multiple training sessions were held for health-care professionals, emphasizing the need to monitor patients with mild hemorrhagic manifestations or hemoconcentration and to insure prompt administration of intravenous fluids. Reported by: C Feliciano de Melecio, MD; H Horta; R Barea, A Casta-Velez, MSS, A Ayala, MPH, C Vargas-Nunez, Div of Epidemiology, C Deseda, MD, State Epidemiologist, Puerto Rico Dept of Health; R Hunter-Mellado, MD, J Morales-Morales, MD, I Figueroa, MPH, Hospital San Pablo; O Reyes, Hospital Hermanos Melendez, Bayamon; B Munoz, MD, San Juan City Hospital; MA Mercado, Hospital del Maestro; L D¤vila, Hospital Auxilio Mutuo; E German, Hospital de Ninos San Jorge; Puerto Rico Association of Epidemiologists, San Juan. Dengue Br and Arbovirus Diseases Br, Div of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: In Puerto Rico, three dengue serotypes (DEN-1, DEN-2, and DEN-4) had circulated in the population from 1985 to 1997. Dengue typically occurs in a seasonal pattern, with minimal occurrence from March to June and a transmission peak from September to November. The number of reported cases during the last 5 nonepidemic years (1992, 1993, 1995, 1996, and 1997) ranged from 4645 to 11,078 (an average rate of 2.0 cases per 1000 population). In 1994, 23,693 cases were reported (6.7 per 1000 population). Although the introduction of a new serotype is one of the strongest determinants of an epidemic, the predominant viruses in the 1998 epidemic are DEN-4 and DEN-1, both of which have been present in Puerto Rico since 1981. Nevertheless, because of the 20-year absence of DEN-3, a large number of island residents are at risk for infection. Reporting was suspended briefly because of Hurricane Georges; however, preliminary analysis of surveillance data suggests that the epidemic peaked at the end of August, and dengue incidence is now decreasing. Although the findings of a large survey in Puerto Rico in 1996 found high levels of awareness about dengue and the Ae. aegypti mosquito, most of the population is not taking action to control this vector (7). The principal barriers to action are lack of knowledge about how to locate and eliminate containers that could serve as larval habitats, the absence of external motivators to prompt the behavior, and the lack of positive feedback and other factors to encourage the public to carry out the necessary actions (7). Since the announcement of the initial phases of the epidemic in July, the PRDH, CDC, civic groups, and private organizations have initiated a public education campaign for mayors, Civil Defense and community leaders, and the public at large, addressing these issues and emphasizing the presence of DEN-3 on the island. Ae. aegypti is an urban mosquito usually found in or near human dwellings (e.g., closets, bathrooms, behind curtains, and under beds). The species bites preferentially, although not exclusively, in the early morning and the afternoon (8). There is no vaccine to prevent dengue. Residents or persons traveling to areas with endemic disease can reduce exposure to mosquito bites by using mosquito repellents on exposed skin and clothing and remaining in well-screened or air-conditioned areas. Aggressive community action to eliminate mosquito breeding sites, in coordination with local and state government activities, appears to be the only effective and permanent method to prevent or control dengue transmission. Dengue should be considered by physicians in the differential diagnosis of all patients who present with fever and a recent history of travel to a tropical area. Acetaminophen products are recommended for managing fever; acetylsalicylic acid and nonsteroidal anti-inflammatory agents (i.e., aspirin and ibuprofen) should be avoided because of their anticoagulant properties. For diagnosis, acute and convalescent serum samples should be obtained and sent through state or territorial health department laboratories to CDC's Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Infectious Diseases, 2 Calle Casia, San Juan, PR 00921-3200; telephone (787) 766-5181; fax (787) 766-6596; e-mail, his1@cdc.gov. Serum samples should be accompanied by clinical and epidemiologic information, including date of disease onset, date of collection of sample, and detailed recent travel history. References
Figure_1 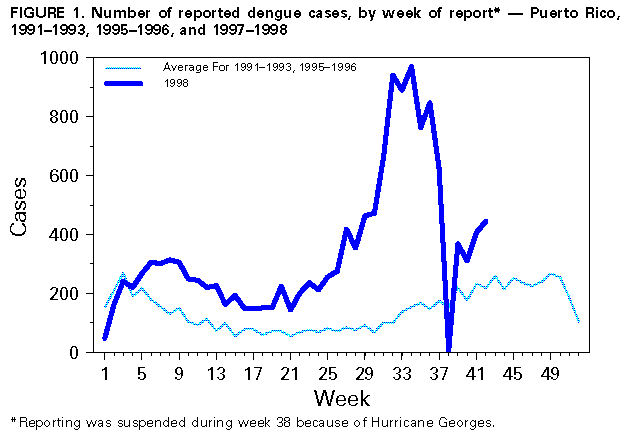 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/12/98 |
|||||||||
This page last reviewed 5/2/01
|