 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and AdolescentsThe material in this report was prepared for publication by: Sharilyn K. Stanley, M.D. National Institute of Allergy and Infectious Diseases National Institutes of Health in collaboration with Jonathan E. Kaplan, M.D. National Center for Infectious Diseases Division of AIDS, STD, and TB Laboratory Research and National Center for HIV, STD, and TB Prevention Division of HIV/AIDS Prevention Surveillance, and Epidemiology Members of the Panel on Clinical Practices for Treatment of HIV Infection Anthony Fauci, M.D. (Co-Chair) Fred Gordin, M.D. National Institutes of Health Veterans Administration Medical Bethesda, MD Center Washington, DC John Bartlett, M.D. (Co-Chair) Johns Hopkins University Wayne Greaves, M.D. Baltimore, MD Howard University Washington, DC Eric Goosby, M.D. (Convener) DHHS Mark Harrington Washington, DC Treatment Action Group New York, NY Mark Smith, M.D. (Co-Convener) Henry J. Kaiser Family Foundation John Henning, Ph.D. Menlo Park, CA American Medical Association Chicago, IL Sophia Chang, M.D., M.P.H. Henry J. Kaiser Family Foundation Martin Hirsch, M.D. Menlo Park, CA Massachusetts General Hospital Boston, MA Jean Anderson, M.D. Johns Hopkins University Richard Marlink, M.D. Baltimore, MD Harvard AIDS Institute Cambridge, MA Rodney Armstead, M.D. Watts Health Foundation, Inc. Celia Maxwell, M.D. Inglewood, CA AIDS Education and Training Center Washington, DC
AIDS University of Pittsburgh Washington, DC Pittsburgh, PA David Barr, J.D. David Nash, M.D. Forum for Collaborative HIV Research Thomas Jefferson University Washington, DC Philadelphia, PA Samuel Bozzette, M.D., Ph.D. Sallie Perryman SDVA Medical Center New York State Department of Health San Diego, CA New York, NY Spencer Cox Robert Schooley, M.D. Treatment Action Group University of Colorado New York, NY Denver, CO Martin Delaney Renslow Sherer, M.D. Project Cook County HIV Primary Care Center San Francisco, CA Chicago, IL Stephen Spector, M.D. Paul Volberding, M.D. University of California University of California San Diego, La Jolla, CA San Francisco, CA Gabriel Torres, M.D. St. Vincent's Hospital New York, NY Participants from the Department of Health and Human Services Barbara Brady Henry Masur, M.D. Office of HIV/AIDS Policy National Institutes of Health Washington, DC Bethesda, MD Elaine Daniels, M.D., Ph.D. Lynne Mofenson, M.D. Office of HIV/AIDS Policy National Institutes of Health Washington, DC Bethesda, MD David Feigel, M.D., M.P.H. Joseph O'Neill, M.D., M.P.H. U.S. Food and Drug Administration Health Resources and Services Bethesda, MD Administration Rockville, MD Mark Feinberg, M.D., Ph.D. National Institutes of Health Lucille Perez, M.D. Bethesda, MD Substance Abuse and Mental Health Services Administration Helene Gayle, M.D., M.P.H. Rockville, MD Centers for Disease Control and Prevention Richard Riseberg, J.D. Atlanta, GA Office of the Secretary Department of Health and Human T. Randolph Graydon Services Health Care Financing Administration Rockville, MD Baltimore, MD Samuel Shekar, M.D., M.P.H. Jonathan Kaplan, M.D. Health Care Financing Centers for Disease Control and Administration Prevention Rockville, MD Atlanta, GA Sharilyn Stanley, M.D. Abe Macher, M.D. National Institutes of Health Health Resources and Services Bethesda, MD Administration Bethesda, MD Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents * Summary With the development and FDA approval of an increasing number of antiretroviral agents, decisions regarding the treatment of HIV-infected persons have become complex; and the field continues to evolve rapidly. In 1996, the Department of Health and Human Services and the Henry J. Kaiser Family Foundation convened the Panel on Clinical Practices for the Treatment of HIV to develop guidelines for the clinical management of HIV-infected persons. This report includes the guidelines developed by the Panel regarding the use of laboratory testing in initiating and managing antiretroviral therapy, considerations for initiating therapy, whom to treat, what regimen of antiretroviral agents to use, when to change the antiretroviral regimen, treatment of the acutely HIV-infected person, special considerations in adolescents, and special considerations in pregnant women. Viral load and CD4+ T cell testing should ideally be performed twice before initiating or changing an antiretroviral treatment regimen. All patients who have advanced or symptomatic HIV disease should receive aggressive antiretroviral therapy. Initiation of therapy in the asymptomatic person is more complex and involves consideration of multiple virologic, immunologic, and psychosocial factors. In general, persons who have less than 500 CD4+ T cells per mm3 should be offered therapy; however, the strength of the recommendation to treat should be based on the patient's willingness to accept therapy as well as the prognosis for AIDS-free survival as determined by the HIV RNA copy per mL of plasma and the CD4+ T cell count. Persons who have greater than 500 CD4+ T cells per mm3 can be observed or can be offered therapy; again, risk of progression to AIDS, as determined by HIV RNA viremia and CD4+ T cell count, should guide the decision to treat. Once the decision to initiate antiretroviral therapy has been made, treatment should be aggressive with the goal of maximal viral suppression. In general, a protease inhibitor and two non-nucleoside reverse transcriptase inhibitors should be used initially. Other regimens may be utilized but are considered less than optimal. Many factors, including reappearance of previously undetectable HIV RNA, may indicate treatment failure. Decisions to change therapy and decisions regarding new regimens must be carefully considered; there are minimal clinical data to guide these decisions. Patients with acute HIV infection should probably be administered aggressive antiretroviral therapy; once initiated, duration of treatment is unknown and will likely need to continue for several years, if not for life. Special considerations apply to adolescents and pregnant women and are discussed in detail. INTRODUCTION These guidelines were developed by the Panel on Clinical Practices for Treatment of HIV Infection, convened by the Department of Health and Human Services (DHHS) and the Henry J. Kaiser Family Foundation. The guidelines contain recommendations for the clinical use of antiretroviral agents in the treatment of adults and adolescents (defined in Considerations for Antiretroviral Therapy in the HIV-Infected Adolescent) who are infected with the human immunodeficiency virus (HIV). Guidance for the use of antiretroviral treatment in pediatric HIV infection is not contained in this report. Although the pathogenesis of HIV infection and the general virologic and immunologic principles underlying the use of antiretroviral therapy are similar for all HIV-infected persons, unique therapeutic and management considerations apply to HIV-infected children. In recognition of these differences, a separate set of guidelines will address pediatric-specific issues related to antiretroviral therapy. These guidelines are intended for use by physicians and other health-care providers who use antiretroviral therapy to treat HIV-infected adults and adolescents. The recommendations contained herein are presented in the context of and with reference to the first section of this report, Principles of Therapy for HIV Infection, formulated by the National Institutes of Health (NIH) Panel to Define Principles of Therapy of HIV Infection. Together, these reports provide the pathogenesis-based rationale for therapeutic strategies as well as practical guidelines for implementing these strategies. Although the guidelines represent the current state of knowledge regarding the use of antiretroviral agents, this field of science is rapidly evolving, and the availability of new agents or new clinical data regarding the use of existing agents will result in changes in therapeutic options and preferences. The Antiretroviral Working Group, a subgroup of the Panel, will meet several times a year to review new data; recommendations for changes in this document would then be submitted to the Panel and incorporated as appropriate. Copies of this document and all updates are available from the CDC National AIDS Clearinghouse (1-800-458-5231) and are posted on the Clearinghouse World-Wide Web site (http://www.cdcnac.org). In addition, copies and updates also are available from the HIV/AIDS Treatment Information Service (1-800-448-0440; Fax 301-519-6616; TTY 1-800-243-7012) and on the ATIS World-Wide Web site (http://www.hivatis.org). Readers should consult these web sites regularly for updates in the guidelines. These recommendations are not intended to substitute for the judgment of a physician who is expert in caring for HIV-infected persons. When possible, the treatment of HIV-infected patients should be directed by a physician with extensive experience in the care of these patients. When this is not possible, the physician treating the patient should have access to such expertise through consultations. Each recommendation is accompanied by a rating that includes a letter and a Roman numeral (Table_1), similar to the rating schemes described in previous guidelines on the prophylaxis of opportunistic infections (OIs) issued by the U.S. Public Health Service and the Infectious Diseases Society of America (1). The letter indicates the strength of the recommendation based on the opinion of the Panel, and the Roman numeral rating reflects the nature of the evidence for the recommendation (Table_1). Thus, recommendations based on data from clinical trials with clinical endpoints are differentiated from recommendations based on data derived from clinical trials with laboratory endpoints (e.g., CD4+ T cell count or plasma HIV RNA levels); when clinical trial data are not available, recommendations are based on the opinions of experts familiar with the relevant scientific literature. The majority of current clinical trial data regarding the use of antiretroviral agents has been obtained in trials enrolling predominantly young to middle-aged males. Although current knowledge indicates that women may differ from men in the absorption, metabolism, and clinical effects of certain pharmacologic agents, clinical experience and data available to date do not indicate any substantial sex differences that would modify these guidelines. However, theoretical concerns exist, and the Panel urges continuation of the current efforts to enroll more women in antiretroviral clinical trials so that the data needed to re-evaluate this issue can be gathered expeditiously. This report addresses the following issues: the use of testing for plasma HIV RNA levels (viral load) and CD4+ T cell count; initiating therapy in established HIV infection; initiating therapy in patients who have advanced-stage HIV disease; interruption of antiretroviral therapy; changing therapy and available therapeutic options; the treatment of acute HIV infection; antiretroviral therapy in adolescents; and antiretroviral therapy in the pregnant woman. USE OF TESTING FOR PLASMA HIV RNA LEVELS AND CD4+ T CELL COUNT IN GUIDING DECISIONS FOR THERAPY Decisions regarding either initiating or changing antiretroviral therapy should be guided by monitoring the laboratory parameters of both plasma HIV RNA (viral load) and CD4+ T cell count and by assessing the clinical condition of the patient. Results of these two laboratory tests provide the physician with important information about the virologic and immunologic status of the patient and the risk of disease progression to acquired immunodeficiency syndrome (AIDS) (see Principle 2 in the first section of this report). HIV viral load testing has been approved by the U.S. Food and Drug Administration (FDA) only for the RT-PCR assay (Roche) and only for determining disease prognosis. However, data presented at an FDA Advisory Committee for the Division of Antiviral Drug Products (July 14-15, 1997, Silver Spring, MD) provide further evidence for the utility of viral RNA testing in monitoring therapeutic responses. Multiple analyses of more than 5,000 patients who participated in approximately 18 trials with viral load monitoring demonstrated a reproducible dose-response type association between decreases in plasma viremia and improved clinical outcome based on standard endpoints of new AIDS-defining diagnoses and survival. This relationship was observed over a range of patient baseline characteristics, including pretreatment plasma RNA level, CD4+ T cell count, and prior drug experience. The consensus of the Panel is that viral load testing is the essential parameter in decisions to initiate or change antiretroviral therapies. Measurement of plasma HIV RNA levels (viral load), using quantitative methods, should be performed at the time of diagnosis of HIV infection and every 3-4 months thereafter in the untreated patient (AIII) (Table_2). CD4+ T cell counts should be measured at the time of diagnosis and generally every 3-6 months thereafter (AIII). These intervals between tests are merely recommendations, and flexibility should be exercised according to the circumstances of the individual case. Plasma HIV RNA levels also should be measured immediately prior to and again at 4-8 weeks after initiation of antiretroviral therapy (AIII). This second time point allows the clinician to evaluate the initial effectiveness of therapy because in most patients, adherence to a regimen of potent antiretroviral agents should result in a large decrease (~0.5 to 0.75 log10) in viral load by 4-8 weeks. The viral load should continue to decline over the following weeks, and in most persons it becomes below detectable levels (currently defined as less than 500 RNA copies/mL) by 12-16 weeks of therapy. The speed of viral load decline and the movement toward undetectable are affected by the baseline CD4+ T cell count, the initial viral load, potency of the regimen, adherence, prior exposure to antiretroviral agents, and the presence of any OIs. These individual differences must be considered when monitoring the effect of therapy. However, the absence of a virologic response of the magnitude previously described (i.e., ~0.5 to 0.75 log10 by 4-8 weeks and undetectable by 12-16 weeks) should prompt the physician to reassess patient adherence, rule out malabsorption, consider repeat RNA testing to document lack of response, and/or consider a change in drug regimen. Once the patient is on therapy, HIV RNA testing should be repeated every 3-4 months to evaluate the continuing effectiveness of therapy (AII). With optimal therapy, viral levels in plasma at 6 months should be undetectable (i.e., less than 500 copies of HIV RNA per mL of plasma) (2). If HIV RNA remains above 500 copies/mL in plasma after 6 months of therapy, the plasma HIV RNA test should be repeated to confirm the result, and a change in therapy should be considered according to the guidelines provided in "Considerations for Changing a Failing Regimen" (BIII). More sensitive viral load assays are in development that can quantify HIV RNA down to approximately 50 copies/mL. Preliminary data from clinical trials strongly suggest that lowering plasma HIV RNA to below 50 copies/mL is associated with a more complete and durable viral suppression, compared with reducing HIV RNA to levels between 50-500 copies/mL. However, the clinical significance of these findings is currently unclear. When deciding whether to initiate therapy, the CD4+ T cell count and plasma HIV RNA measurement ideally should be performed on two occasions to ensure accuracy and consistency of measurement (BIII). However, in patients with advanced HIV disease, antiretroviral therapy should generally be initiated after the first viral load measurement is obtained to prevent a potentially deleterious delay in treatment. Although the requirement for two measurements of viral load may place a substantial financial burden on patients or payers, two measurements of viral load should provide the clinician with the best information for subsequent follow-up of the patient. Plasma HIV RNA levels should not be measured during or within 4 weeks after successful treatment of any intercurrent infection, resolution of symptomatic illness, or immunization (see Principle 2). Because differences exist among commercially available tests, confirmatory plasma HIV RNA levels should be measured by the same laboratory using the same technique to ensure consistent results. A substantial change in plasma viremia is considered to be a threefold or 0.5 log10 increase or decrease. A substantial decrease in CD4+ T cell count is a decrease of greater than 30% from baseline for absolute cell numbers and a decrease of greater than 3% from baseline in percentages of cells (3,4). Discordance between trends in CD4+ T cell numbers and plasma HIV RNA levels can occur and was found in 20% of patients in one cohort studied (5). Such discordance can complicate decisions regarding antiretroviral therapy and may be due to several factors that affect plasma HIV RNA testing (see Principle 2). Viral load and trends in viral load are considered to be more informative for guiding decisions regarding antiretroviral therapy than are CD4+ T cell counts; exceptions to this rule do occur, however (see Considerations for Changing a Failing Regimen); when changes in viral loads and CD4+ T cell counts are discordant, expert consultation should be considered. ESTABLISHED HIV INFECTION Patients who have established HIV infection are considered in two arbitrarily defined clinical categories: 1) asymptomatic infection or 2) symptomatic disease (e.g., wasting, thrush, or unexplained fever for greater than or equal to 2 weeks), including AIDS, defined according to the 1993 CDC classification system (6). All patients in the second category should be offered antiretroviral therapy. Considerations for initiating antiretroviral therapy in the first category of patients (i.e., patients who are asymptomatic) are complex and are discussed separately in the following section. However, before initiating therapy in any patient, the following evaluation should be performed:
prevention of OIs, if not already performed (i.e., VDRL, tuberculin skin test, toxoplasma IgG serology, and gynecologic exam with Pap smear), and other tests as clinically indicated (e.g., chest radiograph, hepatitis C virus {HCV} serology, ophthalmologic exam) (AII). Hepatitis B virus (HBV) serology is indicated for a patient who is a candidate for the hepatitis B vaccine or who has abnormal liver function tests (AII); cytomegalovirus (CMV) serology may be useful in certain persons, as discussed in 1997 USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected With the Human Immunodeficiency Virus (1) (BIII). Considerations for Initiating Therapy in the Patient Who Has Asymptomatic HIV Infection It has been demonstrated that antiretroviral therapy provides clinical benefit in HIV-infected persons who have advanced HIV disease and immunosuppression (7-11). Although there is theoretical benefit to treating patients who have CD4+ T cells greater than 500 cells/mm3 (see Principle 3), no long-term clinical benefit of treatment has yet been demonstrated. A major dilemma confronting patients and practitioners is that the antiretroviral regimens currently available that have the greatest potency in terms of viral suppression and CD4+ T cell preservation are medically complex, are associated with several specific side effects and drug interactions, and pose a substantial challenge for adherence. Thus, decisions regarding treatment of asymptomatic, chronically infected persons must balance a number of competing factors that influence risk and benefit. The physician and the asymptomatic patient must consider multiple risks and benefits in deciding when to initiate therapy (Table_3) (see Principle 3). Several factors influence the decision to initiate early therapy: the real or potential goal of maximally suppressing viral replication; preserving immune function; prolonging health and life; decreasing the risk of drug resistance due to early suppression of viral replication with potent therapy; and decreasing drug toxicity by treating the healthier patient. Factors weighing against early treatment in the asymptomatic stable patient include the following: the potential adverse effects of the drugs on quality of life, including the inconvenience of most of the maximally suppressive regimens currently available (e.g., dietary change or large numbers of pills); the potential risk of developing drug resistance despite early initiation of therapy; the potential for limiting future treatment options due to cycling of the patient through the available drugs during early disease; the potential risk of transmission of virus resistant to protease inhibitors and other agents; the unknown durability of effect of the currently available therapies; and the unknown long-term toxicity of some drugs. Thus, the decision to begin therapy in the asymptomatic patient is complex and must be made in the setting of careful patient counseling and education. The factors that must be considered in this decision include the following: 1) the willingness of the individual to begin therapy; 2) the degree of existing immunodeficiency as determined by the CD4+ T cell count; 3) the risk for disease progression as determined by the level of plasma HIV RNA (Table_4; Figure_1); 4) the potential benefits and risks of initiating therapy in asymptomatic persons, as discussed above; and 5) the likelihood, after counseling and education, of adherence to the prescribed treatment regimen. In regard to adherence, no patient should automatically be excluded from consideration for antiretroviral therapy simplyecause he or she exhibits a behavior or other characteristic judged by some to lend itself to noncompliance. The likelihood of patient adherence to a complex drug regimen should be discussed and determined by the individual patient and physician before therapy is initiated. To achieve the level of adherence necessary for effective therapy, providers are encouraged to utilize strategies for assessing and assisting adherence that have been developed in the context of chronic treatment for other serious diseases. Intensive patient education regarding the critical need for adherence should be provided, specific goals of therapy should be established and mutually agreed upon, and a long-term treatment plan should be developed with the patient. Intensive follow-up should take place to assess adherence to treatment and to continue patient counseling to prevent transmission of HIV through sexual contact and injection of drugs. Initiating Therapy in the Patient Who Has Asymptomatic HIV Infection Once the patient and physician have decided to initiate antiretroviral therapy, treatment should be aggressive, with the goal of maximal suppression of plasma viral load to undetectable levels. Recommendations regarding when to initiate therapy and what regimens to use are provided (Table_5 and Table_6). In general, any patient who has less than 500 CD4+ T cells/mm3 or greater than 10,000 (bDNA) or 20,000 (RT-PCR) copies of HIV RNA/mL of plasma should be offered therapy (AII). However, the strength of the recommendation for therapy should be based on the readiness of the patient for treatment and a consideration of the prognosis for risk for progression to AIDS as determined by viral load, CD4+ T cell count (Table_4; Figure_1), and the slope of the CD4+ T cell count decline. The values for bDNA (Table_4; Figure_1, first column or line) are the uncorrected HIV RNA values obtained from the Multicenter AIDS Cohort Study (MACS). It had previously been thought that these values, obtained on stored heparinized plasma specimens, should be multiplied by a factor of two to adjust for an anticipated twofold loss of RNA ascribed to the effects of heparin and delayed processing on the stability of RNA. However, more recent analysis suggests that the reduction ascribed to these factors is less than or equal to 0.2 log, so that no significant correction factor is necessary (Mellors J, personal communication, October 1997). RT-PCR values also are provided (Table_4; Figure_1); comparison of the results obtained from the RT-PCR and bDNA assays, using the manufacturer's controls, consistently indicates that the HIV-1 RNA values obtained by RT-PCR are approximately twice those obtained by the bDNA assay (12). Thus, the MACS values must be multiplied by approximately 2 to be consistent with current RT-PCR values. A third test for HIV RNA, the nucleic acid sequence based amplification (NASBA (R)), is currently used in some clinical settings. However, formulas for converting values obtained from either branched DNA (bDNA) or RT-PCR assays to NASBA (R)-equivalent values cannot be derived from the limited data currently available. Currently, there are two general approaches to initiating therapy in the asymptomatic patient: a) a therapeutically more aggressive approach in which most patients would be treated early in the course of HIV infection due to the recognition that HIV disease is virtually always progressive and b) a therapeutically more cautious approach in which therapy may be delayed because the balance of the risk for clinically significant progression and other factors discussed above are considered to weigh in favor of observation and delayed therapy. The aggressive approach is heavily based on the Principles of Therapy, particularly the principle (see Principle 3) that one should begin treatment before the development of significant immunosuppression and one should treat to achieve undetectable viremia; thus, all patients who have less than 500 CD4+ T cells/mm3 would be started on therapy as would patients who have higher CD4+ T cell numbers and plasma viral load greater than 10,000 (bDNA) or 20,000 (RT-PCR) (Table_5). The more conservative approach to the initiation of therapy in the asymptomatic person would delay treatment of the patient who has less than 500 CD4+ T cells/mm3 and low levels of viremia and who has a low risk for rapid disease progression (Table_4); careful observation and monitoring would continue. Patients who have CD4+ T cell counts greater than 500/mm3 would also be observed, except those who are at substantial risk for rapid disease progression because of a high viral load. For example, the patient who has 60,000 (RT-PCR) or 30,000 (bDNA) copies of HIV RNA/mL, regardless of CD4+ T cell count, has a high probability of progressing to an AIDS-defining complication of HIV disease within 3 years (32.6% if CD4+ T cells are greater than 500/mm3) and should clearly be encouraged to initiate antiretroviral therapy. Conversely, a patient who has 18,000 copies of HIV RNA/mL of plasma, measured by RT-PCR, and a CD4+ T cell count of 410/mm3, has a 5.9% chance of progressing to an AIDS-defining complication of HIV infection in 3 years (Table_4). The therapeutically aggressive physician would recommend treatment for this patient to suppress the ongoing viral replication that is readily detectable; the therapeutically more conservative physician would discuss the possibility of initiation of therapy but recognize that a delay in therapy because of the balance of considerations previously discussed also is reasonable. In either case, the patient should make the final decision regarding acceptance of therapy following discussion with the health-care provider regarding specific issues relevant to his/her own clinical situation. When initiating therapy in the patient who has never been administered antiretroviral therapy, one should begin with a regimen that is expected to reduce viral replication to undetectable levels (AIII). Based on the weight of experience, the preferred regimen to accomplish this consists of two nucleoside reverse transcriptase inhibitors (NRTIs) and one potent protease inhibitor (PI) (Table_6). Alternative regimens have been employed; these regimens include ritonavir and saquinavir (with one or two NRTIs) or nevirapine as a substitute for the PI. Dual PI therapy with ritonavir and saquinavir (hard-gel formulation), without an NRTI, appears to be potent in suppressing viremia below detectable levels and has convenient twice-daily dosing; however, the safety of this combination has not been fully established according to FDA guidelines. Also, this regimen has not been directly compared with the proven regimens of two NRTIs and a PI; thus, the Panel recommends that at least one additional NRTI be used when the physician elects to use two PIs as initial therapy. Substituting nevirapine for the PI, or using two NRTIs alone, does not achieve the goal of suppressing viremia to below detectable levels as consistently as does combination treatment with two NRTIs and a PI and should be used only if more potent treatment is not possible. However, some experts consider that there currently are insufficient data to choose between a three-drug regimen containing a PI and one containing nevirapine in the patient who has never been administered therapy; further studies are pending. Other regimens using two PIs or a PI and a non-nucleoside reverse transcriptase inhibitor (NNRTI) as initial therapy are currently in clinical trials with data pending. Of the two available NNRTIs, clinical trials support a preference for nevirapine over delavirdine based on results of viral load assays. Although 3TC is a potent NRTI when used in combination with another NRTI, in situations in which suppression of virus replication is not complete, restance to 3TC develops rapidly (13,14). Therefore, the optimal use for this agent is as part of a three-or-more drug combination that has a high probability of complete suppression of virus replication. Other agents in which a single genetic mutation can confer drug resistance (e.g., the NNRTIs nevirapine and delavirdine) also should be used in this manner. Use of antiretroviral agents as monotherapy is contraindicated (DI), except when no other options exist or during pregnancy to reduce perinatal transmission. When initiating antiretroviral therapy, all drugs should be started simultaneously at full dose with the following three exceptions: dose escalation regimens are recommended for ritonavir, nevirapine, and, in some cases, ritonavir plus saquinavir. Detailed information comparing the different NRTIs, the NNRTIs, the PIs, and drug interactions between the PIs and other agents is provided (Table_7, Table_8, Table_9, Table_10, Table_11, Table_12). Particular attention should be paid to drug interactions between the PIs and other agents (Table_9, Table_10, Table_11, Table_12), as these are extensive and often require dose modification or substitution of various drugs. Toxicity assessment is an ongoing process; assessment at least twice during the first month of therapy and every 3 months thereafter is a reasonable management approach. Initiating Therapy in Patients Who Have Advanced-Stage HIV Disease All patients diagnosed as having advanced HIV disease, which is defined as any condition meeting the 1993 CDC definition of AIDS (6), should be treated with antiretroviral agents regardless of plasma viral levels (AI). All patients who have symptomatic HIV infection without AIDS, defined as the presence of thrush or unexplained fever, also should be treated. Special Considerations in the Patient Who Has Advanced-Stage HIV Disease Some patients with OIs, wasting, dementia, or malignancy are first diagnosed with HIV infection at this advanced stage of disease. All patients who have advanced HIV disease should be treated with antiretroviral therapy. When the patient is acutely ill with an OI or other complication of HIV infection, the clinician should consider clinical issues (e.g., drug toxicity, ability to adhere to treatment regimens, drug interactions, and laboratory abnormalities) when determining the timing of initiation of antiretroviral therapy. Once therapy is initiated, a maximally suppressive regimen (e.g., two NRTIs and a PI) should be used (Table_6). Advanced-stage patients being maintained on an antiretroviral regimen should not have the therapy discontinued during an acute OI or malignancy, unless concerns exist regarding drug toxicity, intolerance, or drug interactions. Patients who have progressed to AIDS often are treated with complicated combinations of drugs, and the clinician and patient should be alert to the potential for multiple drug interactions. Thus, the choice of which antiretroviral agents to use must be made with consideration given to potential drug interactions and overlapping drug toxicities (Table_7, Table_8, Table_9, Table_10, Table_11, Table_12). For instance, the use of rifampin to treat active tuberculosis is problematic in a patient who is being administered a PI, which adversely affects the metabolism of rifampin but is frequently needed to effectively suppress viral replication in these advanced patients. Conversely, rifampin lowers the blood level of PIs, which may result in suboptimal antiretroviral therapy. Although rifampin is contraindicated or not recommended for use with all of the PIs, the clinician might consider using a reduced dose of rifabutin (Table_8, Table_9, Table_10, Table_11); this topic is discussed in greater detail elsewhere (15). Other factors complicating advanced disease are wasting and anorexia, which may prevent patients from adhering to the dietary requirements for efficient absorption of certain protease inhibitors. Bone marrow suppression associated with ZDV and the neuropathic effects of ddC, d4T and ddI may combine with the direct effects of HIV to render the drugs intolerable. Hepatotoxicity associated with certain PIs may limit the use of these drugs, especially in patients who have underlying liver dysfunction. The absorption and half life of certain drugs may be altered by antiretroviral agents, particularly the PIs and NNRTIs whose metabolism involves the hepatic cytochrome p450 (CYP450) enzymatic pathway. Some of these PIs and NNRTIs (i.e., ritonavir, indinavir, saquinavir, nelfinavir, and delavirdine) inhibit the CYP450 pathway; others (e.g., nevirapine) induce CYP450 metabolism. CYP450 inhibitors have the potential to increase blood levels of drugs metabolized by this pathway. Adding a CYP450 inhibitor can sometimes improve the pharmacokinetic profile of selected agents (e.g., adding ritonavir therapy to the hard-gel formulation of saquinavir) as well as contribute an additive antiviral effect; however, these interactions also can result in life-threatening drug toxicity (Table_10, Table_11, Table_12). As a result, health-care providers should inform their patients of the need to discuss any new drugs, including over-the-counter agents and alternative medications, that they may consider taking, and careful attention should be given to the relative risk versus benefits of specific combinations of agents. Initiation of potent antiretroviral therapy often is associated with some degree of recovery of immune function. In this setting, patients who have advanced HIV disease and subclinical opportunistic infections (e.g., mycobacterium avium intracellulare {MAI} or CMV) may develop a new immunologic response to the pathogen, and, thus, new symptoms may develop in association with the heightened immunologic and/or inflammatory response. This should not be interpreted as a failure of antiretroviral therapy, and these newly presenting OIs should be treated appropriately while maintaining the patient on the antiretroviral regimen. Viral load measurement is helpful in clarifying this association. INTERRUPTION OF ANTIRETROVIRAL THERAPY There are multiple reasons for temporary discontinuation of antiretroviral therapy, including intolerable side effects, drug interactions, first trimester of pregnancy when the patient so elects, and unavailability of drug. There are no currently available studies and therefore no reliable estimate of the number of days, weeks or months that constitute a clinically important interruption of one or more components of a therapeutic regimen that would increase the likelihood of drug resistance. If any antiretroviral medication has to be discontinued for an extended time, clinicians and patients should be aware of the theoretical advantage of stopping all antiretroviral agents simultaneously, rather than continuing one or two agents, to minimize the emergence of resistant viral strains (see Principle 4). CHANGING A FAILING REGIMEN Considerations for Changing a Failing Regimen The decision to change regimens should be approached with careful consideration of several complex factors. These factors include recent clinical history and physical examination; plasma HIV RNA levels measured on two separate occasions; absolute CD4+ T cell count and changes in these counts; remaining treatment options in terms of potency, potential resistance patterns from prior antiretroviral therapies, and potential for adherence/tolerance; assessment of adherence to medications; and psychological preparation of the patient for the implications of the new regimen (e.g., side effects, drug interactions, dietary requirements and possible need to alter concomitant medications) (see Principle 7). Failure of a regimen may occur for many reasons: initial viral resistance to one or more agents, altered absorption or metabolism of the drug, multidrug pharmacokinetics that adversely affect therapeutic drug levels, and poor patient adherence to a regimen due to either poor compliance or inadequate patient education about the therapeutic agents. In regard to the last issue, the health-care provider should carefully assess patient adherence before changing antiretroviral therapy; health-care workers involved in the care of the patient (e.g., the case manager or social worker) may be helpful in this evaluation. Clinicians should be aware of the prevalence of mental health disorders and psychoactive substance use disorders in certain HIV-infected persons; inadequate mental health treatment services may jeopardize the ability of these persons to adhere to their medical treatment. Proper identification of and intervention in these mental health disorders can greatly enhance adherence to medical HIV treatment. It is important to distinguish between the need to change therapy because of drug failure versus drug toxicity. In the latter case, it is appropriate to substitute one or more alternative drugs of the same potency and from the same class of agents as the agent suspected to be causing the toxicity. In the case of drug failure where more than one drug had been used, a detailed history of current and past antiretroviral medications, as well as other HIV-related medications, should be obtained. Optimally and when possible, the regimen should be changed entirely to drugs that have not been taken previously. With triple combinations of drugs, at least two and preferably three new drugs must be used; this recommendation is based on the current understanding of strategies to prevent drug resistance (see Principles 4 and 5). Assays to determine genotypic resistance are commercially available; however, these have not undergone field testing to demonstrate clinical utility and are not approved by the FDA. The Panel does not recommend these assays for routine use at present. The following three categories of patients should be considered with regard to a change in therapy: 1) persons who are receiving incompletely suppressive antiretroviral therapy with single or double nucleoside therapy and with detectable or undetectable plasma viral load; 2) persons who have been on potent combination therapy, including a PI, and whose viremia was initially suppressed to undetectable levels but has again become detectable; and 3) persons who have been on potent combination therapy, including a PI, and whose viremia was never suppressed to below detectable limits. Although persons in these groups should have treatment regimens changed to maximize the chances of durable, maximal viral RNA suppression, the first group may have more treatment options because they are PI naive. Criteria for Changing Therapy The goal of antiretroviral therapy, which is to improve the length and quality of the patient's life, is likely best accomplished by maximal suppression of viral replication to below detectable levels (currently defined as less than 500 copies/mL) sufficiently early to preserve immune function. However, this reduction cannot always be achieved with a given therapeutic regimen, and frequently regimens must be modified. In general, the plasma HIV RNA level is the most important parameter to consider in evaluating response to therapy, and increases in levels of viremia that are substantial, confirmed, and not attributable to intercurrent infection or vaccination indicate failure of the drug regimen, regardless of changes in the CD4+ T cell counts. Clinical complications and sequential changes in CD4+ T cell count may complement the viral load test in evaluating a response to treatment. Specific criteria that should prompt consideration for changing therapy include the following:
recognition of the still limited choice of available agents and the knowledge that a decision to change may reduce future treatment options for the patient (see Principle 7). This consideration may influence the physician to be somewhat more conservative when deciding to change therapy. Consideration of alternative options should include potency of the substituted regimen and probability of tolerance of or adherence to the alternative regimen. Clinical trials have demonstrated that partial suppression of virus is superior to no suppression of virus. However, some physicians and patients may prefer to suspend treatment to preserve future options or because a sustained antiviral effect cannot be achieved. Referral to or consultation with an experienced HIV clinician is appropriate when the clinician is considering a change in therapy. When possible, patients who require a change in an antiretroviral regimen but without treatment options that include using currently approved drugs should be referred for consideration for inclusion in an appropriate clinical trial. Therapeutic Options When Changing Antiretroviral Therapy Recommendations for changes in treatment differ according to the indication for the change. If the desired virologic objectives have been achieved in patients who have intolerance or toxicity, a substitution should be made for the offending drug, preferably with an agent in the same class with a different toxicity or tolerance profile. If virologic objectives have been achieved but the patient is receiving a regimen not in the preferred category (e.g., two NRTIs or monotherapy), there is the option either to continue treatment with careful monitoring of viral load or to add drugs to the current regimen to comply with preferred treatment regimens. Most experts consider that treatment with regimens not in the preferred category is associated with eventual failure and recommend the latter tactic. At present, few clinical data are available to support specific strategies for changing therapy in patients who have failed the preferred regimens that include PIs; however, several theoretical considerations should guide decisions. Because of the relatively rapid mutability of HIV, viral strains that are resistant to one or more agents often emerge during therapy, particularly when viral replication has not been maximally suppressed. Of major concern is recent evidence of broad cross-resistance among the class of PIs. Evidence indicates that viral strains that become resistant to one PI will have reduced susceptibility to most or all other PIs. Thus, the likelihood of success of a subsequently administered PI + two NRTI regimen, even if all drugs are different from the initial regimen, may be limited, and many experts would include two new PIs in the subsequent regimen. Some of the most important guidelines to follow when changing a patient's antiretroviral therapy are summarized (Table_13), and some of the treatment options available when a decision has been made to change the antiretroviral regimen are outlined (Table_14). Limited data exist to suggest that any of these alternative regimens will be effective (Table_14), and careful monitoring and consultation with an expert in the care of such HIV-infected patients is desirable. A change in regimen because of treatment failure should ideally involve complete replacement of the regimen with different drugs to which the patient is naive. This typically would include the use of two new NRTIs and one new PI or NNRTI, two PIs with one or two new NRTIs, or a PI combined with an NNRTI. Dose modifications may be required to account for drug interactions when using combinations of PIs or a PI and NNRTI (Table_12). In some persons, these options are not possible because of prior antiretroviral use, toxicity, or intolerance. In the clinically stable patient who has detectable viremia for whom an optimal change in therapy is not possible, it may be prudent to delay changing therapy in anticipation of the availability of newer and more potent agents. It is recommended that the decision to change therapy and design a new regimen should be made with assistance from a clinician experienced in the treatment of HIV infected patients through consultation or referral. ACUTE HIV INFECTION Considerations for Treatment of Patients Who Have Acute HIV Infection Various studies indicate that 50%-90% of patients acutely infected with HIV will experience at least some symptoms of the acute retroviral syndrome (Table_15) and can thus be identified as candidates for early therapy (16-19). However, acute HIV infection is often not recognized in the primary-care setting because of the similarity of the symptom complex with those of the "flu" or other common illnesses. Also, acute primary infection may occur without symptoms. Physicians should maintain a high level of suspicion for HIV infection in all patients with a compatible clinical syndrome (Table_15) and should obtain appropriate laboratory confirmation. Information regarding treatment of acute HIV infection from clinical trials is limited. There is evidence for a short-term effect of therapy on viral load and CD4+ T cell counts (20), but there are as yet no outcome data demonstrating a clinical benefit of antiretroviral treatment of primary HIV infection. Clinical trials completed to date also have been limited by small sample sizes, short duration of follow-up, and often by the use of treatment regimens that have suboptimal antiviral activity by current standards. However, results from these studies generally support antiretroviral treatment of acute HIV infection. Ongoing clinical trials are addressing the question of the long-term clinical benefit of more potent treatment regimens. The theoretical rationale for early intervention (see Principle 10) is fourfold:
HIV infection is based on theoretical considerations, and the potential benefits, described above, should be weighed against the potential risks (see below). Most experts endorse treatment of acute HIV infection based on the theoretical rationale, limited but supportive clinical trial data, and the experience of HIV clinicians. The risks associated with therapy for acute HIV infection include adverse effects on quality of life resulting from drug toxicities and dosing constraints; the potential, if therapy fails to effectively suppress viral replication, for the development of drug resistance that may limit future treatment options; and the potential need for continuing therapy indefinitely. These considerations are similar to those for initiating therapy in the asymptomatic patient (see Considerations in Initiating Therapy in the Asymptomatic HIV-infected Patient). Deciding Whom to Treat During Acute HIV Infection Many experts would recommend antiretroviral therapy for all patients who demonstrate laboratory evidence of acute HIV infection (AII). Such evidence includes HIV RNA in plasma that can be detected by using sensitive PCR or bDNA assays together with a negative or indeterminate HIV antibody test. Although measurement of plasma HIV RNA is the preferable method of diagnosis, a test for p24 antigen may be useful when RNA testing is not readily available. However, a negative p24 antigen test does not rule out acute infection. When suspicion for acute infection is high (e.g., as in a patient who has a report of recent risk behavior in association with suggestive symptoms and signs {Table_15}), a test for HIV RNA should be performed (BII). ** Persons may or may not have symptoms of the acute retroviral syndrome. Viremia occurs acutely after infection before the detection of a specific immune response; an indeterminate antibody test may occur when a person is in the process of seroconversion. Apart from patients who have acute primary HIV infection, many experts also would consider therapy for patients in whom seroconversion has been documented to have occurred within the previous 6 months (CIII). Although the initial burst of viremia in infected adults has usually resolved by 2 months, treatment during the 2-6-month period after infection is based on the likelihood that virus replication in lymphoid tissue is still not maximally contained by the immune system during this time. Decisions regarding therapy for patients who test antibody positive and who believe the infection is recent but for whom the time of infection cannot be documented should be made using the Asymptomatic HIV Infection algorithm mentioned previously (CIII). No patient should be treated for HIV infection until the infection is documented, except in the setting of post-exposure prophylaxis of health-care workers with antiretroviral agents (21) ***. All patients without a formal medical record of a positive HIV test (e.g., persons who have tested positive by available home testing kits) should be tested by both the ELISA and an established confirmatory test (e.g., the Western Blot) to document HIV infection (AI). Treatment Regimen for Primary HIV Infection Once the physician and patient have decided to use antiretroviral therapy for primary HIV infection, treatment should be implemented with the goal of suppressing plasma HIV RNA levels to below detectable levels (AIII). The weight of current experience suggests that the therapeutic regimen for acute HIV infection should include a combination of two NRTIs and one potent PI (AII). Although most experience to date with PIs in the setting of acute HIV infection has been with ritonavir, indinavir or nelfinavir (2,22-24), insufficient data are available to make firm conclusions regarding specific drug recommendations. Potential combinations of agents available are much the same as those used in established infection (Table_6). These aggressive regimens may be associated with several disadvantages (e.g., drug toxicity, large numbers of pills, cost of drugs, and the possibility of developing drug resistance that may limit future options); the latter is likely if virus replication is not adequately suppressed or if the patient has been infected with a viral strain that is already resistant to one or more agents. The patient should be carefully counseled regarding these potential limitations and individual decisions made only after weighing the risks and sequelae of therapy against the theoretical benefit of treatment. Any regimen that is not expected to maximally suppress viral replication is not considered appropriate for treating the acutely HIV-infected person (EIII) because a) the ultimate goal of therapy is suppression of viral replication to below the level of detection, b) the benefits of therapy are based primarily on theoretical considerations, and c) long-term clinical outcome benefit has not been documented. Additional clinical studies are needed to delineate further the role of antiretroviral therapy in the primary infection period. Patient Follow-up Testing for plasma HIV RNA levels and CD4+ T cell count and toxicity monitoring should be performed as previously described in Use of Testing for Plasma HIV RNA levels and CD4+ T Cell Count in Guiding Decisions for Therapy, that is, on initiation of therapy, after 4 weeks, and every 3-4 months thereafter (AII). Some experts suggest that testing for plasma HIV RNA levels at 4 weeks is not helpful in evaluating the effect of therapy for acute infection because viral loads may be decreasing from peak viremia levels even in the absence of therapy. Duration of Therapy for Primary HIV Infection Once therapy is initiated, many experts would continue to treat the patient with antiretroviral agents indefinitely because viremia has been documented to reappear or increase after discontinuation of therapy (CII). However, some experts would treat for one year and then reevaluate the patient with CD4+ T cell determinations and quantitative HIV RNA measurements. The optimal duration and composition of therapy are unknown, and ongoing clinical trials are expected to provide data relevant to these issues. The difficulties inherent in determining the optimal duration and composition of therapy initiated for acute infection should be considered when first counseling the patient regarding therapy. CONSIDERATIONS FOR ANTIRETROVIRAL THERAPY IN THE HIV-INFECTED ADOLESCENT HIV-infected adolescents who were infected through sexual contact or through injecting-drug use during adolescence appear to follow a clinical course that is more similar to HIV disease in adults than in children. In contrast, adolescents who were infected perinatally or through blood products as young children have a unique clinical course that may differ from other adolescents and long-term surviving adults. Currently, most HIV-infected adolescents were infected through sexual contact during the adolescent period and are in a relatively early stage of infection, making them ideal candidates for early intervention. Puberty is a time of somatic growth and hormonally mediated changes, with females developing more body fat and males more muscle mass. Although theoretically these physiologic changes could affect drug pharmacology, particularly in the case of drugs with a narrow therapeutic index that are used in combination with protein-bound medicines or hepatic enzyme inducers or inhibitors, no clinically substantial impact of puberty on the use of NRTIs has been observed. Clinical experience with PIs and NNRTIs has been limited. Thus, it is currently recommended that medications used to treat HIV and OIs in adolescents should be administered in a dosage based on Tanner staging of puberty and not specific age. Adolescents in early puberty (Tanner I-II) should receive doses as recommended in the pediatric guidelines, whereas those in late puberty (Tanner V) should receive doses recommended in the adult guidelines. Youth who are in the midst of their growth spurt (Tanner III females and Tanner IV males) should be closely monitored for medication efficacy and toxicity when choosing adult or pediatric dosing guidelines. CONSIDERATIONS FOR ANTIRETROVIRAL THERAPY IN THE PREGNANT HIV-INFECTED WOMAN Guidelines for optimal antiretroviral therapy and for initiation of therapy in pregnant HIV-infected women should be the same as those delineated for nonpregnant adults (see Principle 8). Thus, the woman's clinical, virologic, and immunologic status should be the primary factor in guiding treatment decisions. However, it must be realized that the potential impact of such therapy on the fetus and infant is unknown. The decision to use any antiretoviral drug during pregnancy should be made by the woman following discussion with her health-care provider regarding the known and unknown benefits and risks to her and her fetus. Long-term follow-up is recommended for all infants born to women who have received antiretroviral drugs during pregnancy. Women who are in the first trimester of pregnancy and who are not receiving antiretroviral therapy may wish to consider delaying initiation of therapy until after 10-12 weeks' gestation because this is the period of organogenesis when the embryo is most susceptible to potential teratogenic effects of drugs; the risks of antiretroviral therapy to the fetus during that period are unknown. However, this decision should be carefully considered and discussed between the health-care provider and the patient and should include an assessment of the woman's health status and the potential benefits and risks of delaying initiation of therapy for several weeks. If clinical, virologic, or immunologic parameters are such that therapy would be recommended for nonpregnant persons, many experts would recommend initiating therapy, regardless of gestational age. Nausea and vomiting in early pregnancy, which affect the ability to adequately take and absorb oral medications, may be a factor in deciding whether to administer treatment during the first trimester. Some women already receiving antiretroviral therapy may have their pregnancy diagnosed early enough in gestation that concern for potential teratogenicity may lead them to consider temporarily stopping antiretroviral therapy until after the first trimester. Insufficient data exist that either support or refute teratogenic risk of antiretroviral drugs when administered during the first 10-12 weeks' gestation. However, a rebound in viral levels would be anticipated during the period of discontinuation, and this rebound could theoretically be associated with increased risk of early in utero HIV transmission or could potentiate disease progression in the woman (25). Although the effects of all antiretroviral drugs on the developing fetus during the first trimester are uncertain, most experts recommend continuation of a maximally suppressive regimen even during the first trimester. If antiretroviral therapy is discontinued during the first trimester for any reason, all agents should be stopped simultaneously to avoid development of resistance. Once the drugs are reinstituted, they should be introduced simultaneously for the same reason. The choice of which antiretroviral agents to use in pregnant women is subject to unique considerations (see Principle 8). Currently, minimal data are available regarding the pharmacokinetics and safety of antiretroviral agents during pregnancy for drugs other than ZDV. In the absence of data, drug choice needs to be individualized based on discussion with the patient and available data from preclinical and clinical testing of the individual drugs. The FDA pregnancy classification for all currently approved antiretroviral agents and selected other information relevant to the use of antiretroviral drugs in pregnancy is provided (Table_16). The predictive value of in vitro and animal-screening tests for adverse effects in humans is unknown. Many drugs commonly used to treat HIV infection or its consequences may have positive findings on one or more of these screening tests. For example, acyclovir is positive on some in vitro assays for chromosomal breakage and carcinogenicity and is associated with some fetal abnormalities in rats; however, data on human experience from the Acyclovir in Pregnancy Registry indicate no increased risk of birth defects to date in infants with in utero exposure to acyclovir (26). Of the currently approved nucleoside analogue antiretroviral agents, the pharmacokinetics of only ZDV and 3TC have been evaluated in infected pregnant women to date (27,28). Both drugs seem to be well tolerated at the usual adult doses and cross the placenta, achieving concentrations in cord blood similar to those observed in maternal blood at delivery. All the nucleosides except ddI have preclinical animal studies that indicate potential fetal risk and have been classified as FDA pregnancy category C (Table_16); ddI has been classified as category B. In primate studies, all the nucleoside analogues seem to cross the placenta, but ddI and ddC apparently have significantly less placental transfer (fetal to maternal drug ratios of 0.3 to 0.5) than do ZDV, d4T, and 3TC (fetal to maternal drug ratios greater than 0.7) (29). Of the NNRTIs, only nevirapine administered once at the onset of labor has been evaluated in pregnant women. The drug was well tolerated after a single dose and crossed the placenta and achieved neonatal blood concentrations equivalent to those in the mother. The elimination of nevirapine administered during labor in the pregnant women in this study was prolonged (mean half-life following a single dose, 66 hours) compared with nonpregnant persons (mean half-life following a single dose, 45 hours). Data on multiple dosing during pregnancy are not yet available. Delavirdine has not been studied in Phase I pharmacokinetic and safety trials in pregnant women. In premarketing clinical studies, outcomes of seven unplanned pregnancies were reported. Three of these were ectopic pregnancies, and three resulted in healthy live births. One infant was born prematurely, with a small ventricular septal defect, to a patient who had received approximately 6 weeks of treatment with delavirdine and ZDV early in the course of pregnancy. Although studies of combination therapy with protease inhibitors in pregnant HIV-infected women are in progress, no data are currently available regarding drug dosage, safety and tolerance during pregnancy. In mice, indinavir has substantial placental passage; however, in rabbits, little placental passage was observed. Ritonavir has been demonstrated to have some placental passage in rats. There are some special theoretical concerns regarding the use of indinavir late in pregnancy. Indinavir is associated with side effects (hyperbilirubinemia and renal stones) that theoretically could be problematic for the newborn if transplacental passage occurs and the drug is administered shortly before delivery. These side effects are particularly problematic because the immaturity of the metabolic enzyme system of the neonatal liver would likely be associated with prolonged drug half-life leading to extended drug exposure in the newborn that could lead to potential exacerbation of physiologic neonatal hyperbilirubinemia. Because of immature neonatal renal function and the inability of the ne hydration, high drug concentrations and/or delayed elimination in the neonate could result in a higher risk for drug crystallization and renal stone development than observed in adults. These concerns are theoretical and such effects have not been reported; because the half-life of indinavir in adults is short, these concerns may only be relevant if drug is administered near the time of labor. Gestational diabetes is a pregnancy-related complication that can develop in some women; administration of any of the four currently available protease inhibitors has been associated with new onset diabetes mellitus, hyperglycemia, or exacerbation of existing diabetes mellitus in HIV-infected patients (30). Pregnancy is itself a risk factor for hyperglycemia, and it is unknown if the use of protease inhibitors will exacerbate this risk for hyperglycemia. Health-care providers caring for infected pregnant women who are being administered PI therapy should be aware of the possibility of hyperglycemia and closely monitor glucose levels in their patients and instruct their patients on how to recognize the early symptoms of hyperglycemia. To date, the only drug that has been shown to reduce the risk of perinatal HIV transmission is ZDV when administered according to the following regimen: orally administered antenatally after 14 weeks' gestation and continued throughout pregnancy, intravenously administered during the intrapartum period, and administered orally to the newborn for the first 6 weeks of life (31). This chemoprophylactic regimen was shown to reduce the risk for perinatal transmission by 66% in a randomized, double-blind clinical trial, pediatric ACTG 076 (32). Insufficient data are available to justify the substitution of any antiretroviral agent other than ZDV to reduce perinatal HIV transmission; further research should address this question. For the time being, if combination antiretroviral drugs are administered to the pregnant woman for treatment of her HIV infection, ZDV should be included as a component of the antenatal therapeutic regimen whenever possible, and the intrapartum and neonatal ZDV components of the chemoprophylactic regimen should be administered to reduce the risk for perinatal transmission. If a woman is not administered ZDV as a component of her antenatal antiretroviral regimen (e.g., because of prior history of nonlife-threatening ZDV-related severe toxicity or personal choice), intrapartum and newborn ZDV should continue to be recommended; when use of ZDV is contraindicated in the woman, the intrapartum component may be deleted, but the newborn component is still recommended. ZDV and d4T should not be administered together due to potential pharmacologic antagonism. When d4T is a preferred nucleoside for treatment of a pregnant woman, it is recommended that antenatal ZDV not be added to the regimen; however, intrapartum and neonatal ZDV should still be given. The time-limited use of ZDV alone during pregnancy for chemoprophylaxis of perinatal transmission is controversial. The potential benefits of standard combination antiretroviral regimens for treatment of HIV infection should be discussed with and offered to all pregnant HIV-infected women. Some women may wish to restrict exposure of their fetus to antiretroviral drugs during pregnancy but still wish to reduce the risk of transmitting HIV to their infant. For women in whom initiation of antiretroviral therapy for treatment of their HIV infection would be considered optional (e.g., CD4+ count greater than 500/mm3 and plasma HIV RNA less than 10,0000-20,000 RNA copies/mL), time-limited use of ZDV during the second and third trimesters of pregnancy is less likely to induce the development of resistance due to the limited viral replication existing in the patient and the time-limited exposure to the antiretroviral drug. For example, the development of resistance was unusual among the healthy population of women who participated in Pediatric (P)-ACTG 076 (33). The use of ZDV chemoprophylaxis alone during pregnancy might be an appropriate option for these women. However, for women who have more advanced disease and/or higher levels of HIV RNA, concerns about resistance are greater and these women should be counseled that a combination antiretroviral regimen that includes ZDV for reducing transmission risk would be more optimal for their own health than use of ZDV chemoprophylaxis alone. Monitoring and use of HIV-1 RNA for therapeutic decision making during pregnancy should be performed as recommended for nonpregnant persons. Transmission of HIV from mother to infant can occur at all levels of maternal HIV-1 RNA. In untreated women, higher HIV-1 RNA levels correlate with increased transmission risk. However, in ZDV-treated women this relationship is markedly attenuated (32). ZDV is effective in reducing transmission regardless of maternal HIV RNA level. Therefore, the use of the full ZDV chemoprophylaxis regimen, including intravenous ZDV during delivery and the administration of ZDV to the infant for the first 6 weeks of life, alone or in combination with other antiretrovirals, should be discussed with and offered to all infected pregnant women regardless of their HIV-1 RNA level. Health-care providers who are treating HIV-infected pregnant women are strongly encouraged to report cases of prenatal exposure to antiretroviral drugs (either administered alone or in combinations) to the Antiretroviral Pregnancy Registry. The registry collects observational, nonexperimental data regarding antiretroviral exposure during pregnancy for the purpose of assessing potential teratogenicity. Registry data will be used to supplement animal toxicology studies and assist clinicians in weighing the potential risks and benefits of treatment for individual patients. The registry is a collaborative project with an advisory committee of obstetric and pediatric practitioners, staff from CDC and NIH, and staff from pharmaceutical manufacturers. The registry allows the anonymity of patients, and birth outcome follow-up is obtained by registry staff from the reporting physician. Referrals should be directed to Antiretroviral Pregnancy Registry, Post Office Box 13398, Research Triangle Park, NC 27709-3398; telephone (800) 258-4263. CONCLUSION The Panel has attempted to use the advances in current understanding of the pathogenesis of HIV in the infected person to translate scientific principles and data obtained from clinical experience into recommendations that can be used by the clinician and patient to make therapeutic decisions. The recommendations are offered in the context of an ongoing dialogue between the patient and the clinician after having defined specific therapeutic goals with an acknowledgment of uncertainties. It is necessary for the patient to receive a continuum of medical care and services, including social, psychosocial, and nutritional services, with the availability of expert referral and consultation. To achieve the maximal flexibility in tailoring therapy to each patient over the duration of his or her infection, it is imperative that drug formularies allow for all FDA-approved NRTI, NNRTI, and PI as treatment options. The Panel strongly urges industry and the public and private sectors to conduct further studies to allow refinement of these guidelines. Specifically, studies are needed to optimize recommendations for first-line therapy; to define second-line therapy; and to more clearly delineate the reason(s) for treatment failure. The Panel remains committed to revising their recommendations as such new data become available. Acknowledgment The Panel extends special appreciation to Charles Carpenter (Brown University School of Medicine, Providence, RI) for his advice in the development of this document and to Gerry Bally (Health Canada) and Anita Rachlis (Sunnybrook Health Science Centre, University of Toronto, Toronto, Canada) for their participation. The Panel acknowledges the special contributions of Sharilyn Stanley, Barbara Brady, and Elaine Daniels in the preparation of this document. References
* Information included in these guidelines may not represent FDA approval or approved labeling for the particular products or indications in question. Specifically, the terms "safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval. ** Patients diagnosed with HIV infection by HIV RNA testing should have confirmatory testing performed (Table_2). *** Or treatment of neonates born to HIV-infected mothers. Appendices Figure_1 FIGURE 1. Likelihood of developing AIDS within 3 years Table_1 TABLE 1. Rating system for strength of recommendation and quality of evidence supporting the recommendation Table_2 TABLE 2. Indications for plasma HIV RNA testing Table_3 TABLE 3. Risks and benefits of early initiation of antiretroviral therapy in the asymptomatic HIV-infected patient Table_4 TABLE 4. Risk for progression to AIDS-defining illness in a cohort of men who have sex with men, predicted by baseline CD4+ T cell count and viral load Table_5 TABLE 5. Indications for the initiation of antiretroviral therapy in the chronically HIV-infected patient Table_6 TABLE 6. Recommended antiretroviral agents for treatment of established HIV infection Table_7 TABLE 7. Characteristics of nucleoside reverse transcriptase inhibitors (NRTIs) Table_8 TABLE 8. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) Table_9 TABLE 9. Characteristics of protease inhibitors (Pis) Table_10 TABLE 10. Drugs that should not be used with protease inhibitors Table_11 TABLE 11. Drug interactions between protease inhibitors and other drugs; drug interactions requiring dose modifications Table_12 TABLE 12. Drug interactions: protease inhibitors and non-nucleoside reverse transcriptase inhibitors -- effect of drug on levels/dose Table_13 Table 13. Guidelines for changing an antiretroviral regimen for suspected drug failure Table_14 TABLE 14. Possible regimens for patients who have failed antiretroviral therapy: a work in progress* Table_15 TABLE 15. Acute retroviral syndrome: associated signs and symptoms and expected frequency* Table_16 TABLE 16. Preclinical and clinical data relevant to use of antiretrovirals during pregnancy +------------------------------------------------------------------- -------+ | | | Erratum: Vol. 47, No. RR-5 | | ========================== | | | | SOURCE:47(29);619 DATE:Jul 31 1998 | | | | In the MMWR Recommendations and Reports, "Guidelines for the Use of | | Antiretroviral Agents in HIV-Infected Adults and Adolescents," on page | | 43, information was incorrectly presented in the summary section. The | | sentence beginning on the 10th line from the end of the summary should | | read, "In general, a protease inhibitor and two nucleoside reverse | | transcriptase inhibitors should be used initially." | | | +------------------------------------------------------------------- -------+ Figure_1 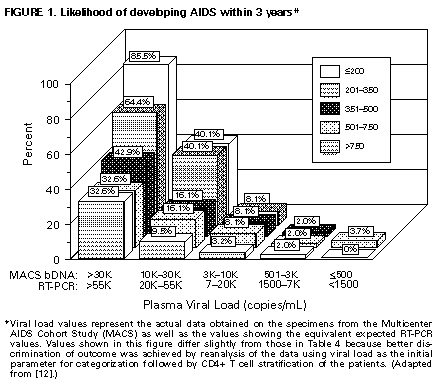 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. TABLE 1. Rating system for strength of recommendation and quality of evidence supporting the recommendation ================================================================================ Category Definition ------------------------------------------------------------------------------ Categories reflecting the strength of each recommendation, A Strong; should always be offered B Moderate; should usually be offered C Optional D Should generally not be offered E Should never be offered Categories reflecting the quality of evidence supporting the recommendation I At least one randomized trial with clinical endpoints II Clinical trials with laboratory endpoints III Expert opinion ------------------------------------------------------------------------------ ================================================================================ Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Indications for plasma HIV RNA testing *
===================================================================================================
Clinical indication Information Use
---------------------------------------------------------------------------------------------
Syndrome consistent with Establishes diagnosis when Diagnosis +
acute HIV infection HIV antibody test is
negative or indeterminate
Initial evaluation of newly Baseline viral load Decision to start or defer
diagnosed HIV infection สset pointฐ therapy
Every 3-4 mos. in patients Changes in viral load Decision to start therapy
not on therapy
4-8 wks. after initiation of Initial assessment of drug Decision to continue or
antiretroviral therapy efficacy change therapy
3-4 mos. after start of Maximal effect of therapy Decision to continue or
therapy change therapy
Every 3-4 mos. in patients Durability of antiretroviral Decision to continue or
on therapy effect change therapy
Clinical event or significant Association with changing Decision to continue,
decline in CD4+ T cells or stable viral load initiate, or change therapy
---------------------------------------------------------------------------------------------
* Acute illness (e.g., bacterial pneumonia, tuberculosis, HSV, PCP) and immunizations can cause
increases in plasma HIV RNA for 2-4 wks.; viral load testing should not be performed during
this time. Plasma HIV RNA results should usually be verified with a repeat determination before
starting or making changes in therapy. HIV RNA should be measured using the same laboratory
and the same assay.
+ Diagnosis of HIV infection determined by HIV RNA testing should be confirmed by standard
methods (e.g., Western blot serology) performed 2-4 mos. after the initial indeterminate or
negative test.
===================================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Risks and benefits of early initiation of antiretroviral therapy in the
asymptomatic HIV-infected patient
-----------------------------------------------------------------------------------------------
Potential Benefits
Control of viral replication and mutation; reduction of viral burden
Prevention of progressive immunodeficiency; potential maintenance or reconstitution of a
normal immune system
Delayed progression to AIDS and prolongation of life
Decreased risk of selection of resistant virus
Decreased risk of drug toxicity
Potential Risks
Reduction in quality of life from adverse drug effects and inconvenience of current maximally
suppressive regimens
Earlier development of drug resistance
Limitation in future choices of antiretroviral agents due to development of resistance
Unknown long-term toxicity of antiretroviral drugs
Unknown duration of effectiveness of current antiretroviral therapies
-----------------------------------------------------------------------------------------------
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Risk for progression to AIDS-defining illness in a cohort of men who have
sex with men, predicted by baseline CD4+ T cell count and viral load *
=============================================================================================
CD4 <=350 % AIDS (AIDS-defining complication) +
Plasma viral load -----------------------------------------
(copies/mL) & No. of
---------------------------------- patients
bDNA RT-PCR in study 3 yrs 6 yrs 9 yrs
--------------------------------------------------------------------------------
<=500 <=1,500 --@ -- -- --
501-3,000 1,501-7,000 30 0 18.8 30.6
3,001-10,000 7,001-20,000 51 8.0 42.2 65.6
10,001-30,000 20,001-55,000 73 40.1 72.9 86.2
>=30,000 >=55,000 174 72.9 92.7 95.6
--------------------------------------------------------------------------------
CD4 351-500 % AIDS (AIDS-defining complication)
Plasma viral load -----------------------------------------
(copies/mL) No. of
---------------------------------- patients
bDNA RT-PCR in study 3 yrs 6 yrs 9 yrs
--------------------------------------------------------------------------------
<=500 <=1,500 -- -- -- --
501-3,000 1,501-7,000 47 4.4 22.1 46.9
3,001-10,000 7,001-20,000 105 5.9 39.8 60.7
10,001-30,000 20,001-55,000 121 15.1 57.2 78.6
>=30,000 >=55,000 121 47.9 77.7 94.4
--------------------------------------------------------------------------------
CD4 >500 % AIDS (AIDS-defining complication)
Plasma viral load -----------------------------------------
(copies/mL) No. of
---------------------------------- patients
bDNA RT-PCR in study 3 yrs 6 yrs 9 yrs
--------------------------------------------------------------------------------
<=500 <=1,500 110 1.0 5.0 10.7
501-3,000 1,501-7,000 180 2.3 14.9 33.2
3,001-10,000 7,001-20,000 237 7.2 25.9 50.3
10,001-30,000 20,001-55,000 202 14.6 47.7 70.6
>=30,000 >=55,000 141 32.6 66.8 76.3
--------------------------------------------------------------------------------
* Data from the Multicenter AIDS Cohort Study (MACS) (12)
+ In this study, AIDS was defined according to the 1987 CDC definition and does not include
asymptomatic persons who have CD4+ T cells <200/mm3
& MACS numbers reflect plasma HIV RNA values obtained by bDNA testing. RT-PCR values are
consistently 2-2.5-fold higher than bDNA values, as indicated.
@ Too few subjects were in the category to provide a reliable estimate of AIDS risk.
=============================================================================================
Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Indications for the initiation of antiretroviral therapy in the chronically
HIV-infected patient
=========================================================================================
Clinical category CD4+ T cell count and HIV RNA Recommendation
-----------------------------------------------------------------------------------
Symptomatic (i.e., Any value Treat
AIDS, thrush,
unexplained fever)
Asymptomatic CD4+ T Cells <500/mm3 Treatment should be
or offered. Strength of
HIV RNA >10,000 (bDNA) recommendation is based
or >20,000 (RT-PCR) on prognosis for
disease-free survival as
shown in Table 4 and
willingness of the patient to
accept therapy. *
Asymptomatic CD4+ T Cells >500/mm3 Many experts would delay
and therapy and observe;
HIV RNA <10,000 (bDNA) however, some experts
or <20,000 (RT-PCR) would treat.
-----------------------------------------------------------------------------------
* Some experts would observe patients whose CD4+ T cell counts are between 350-500/ mm3
and HIV RNA levels <10,000 (bDNA) or <20,000 (RT-PCR).
=========================================================================================
Return to top. Table_6 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 6. Recommended antiretroviral agents for treatment of established HIV
infection
========================================================================================================
Preferred: Strong evidence of clinical benefit and/or sustained suppression of plasma viral load
(2, 34, 35)
One choice each from column A and column B. Drugs are listed in random, not priority, order:
Column A Column B
----------------------------------------
Indinavir (AI) ZDV + ddl (AI)
Nelfinavir (AII) d4T + ddl (AII)
Ritonavir (AI) ZDV + ddC (AI)
Saquinavir-SGC* (AII) ZDV + 3TC& (AI)
Ritonavir + d4T + 3TC& (AII)
Saquinavir-SGC or
HGC+ (BII)
Alternative: Less likely to provide sustained virus suppression; (36-38)
1 NNRTI (Nevirapine)@ + 2 NRTIs (Column B, above) (BII)
Saquinavir-HGC + 2 NRTIs (Column B, above) (BI)
Not generally recommended: Strong evidence of clinical benefit, but initial virus suppression is
not sustained in most patients (39,40)
2 NRTIs (Column B, above) (CI)
Not recommended**: Evidence against use, virologically undesirable, or overlapping toxicities
All monotherapies (DI)
d4T + ZDV (DI)
ddC + ddI++ (DII)
ddC + d4T++ (DII)
ddC + 3TC (DII)
------------------------------------------------------------------------------------------------
* Virologic data and clinical experience with saquinavir-sgc are limited in comparison with
other protease inhibitors.
+ Use of ritonavir 400 mg b.i.d. with saquinavir soft-gel formulation (Fortovase (TM) ) 400 mg b.i.d.
results in similar areas under the curve (AUC) of drug and antiretroviral activity as when
using 400 mg b.i.d. of Invirase (TM) in combination with ritonavir. However, this combination
with Fortovase (TM) has not been extensively studied and gastrointestinal toxicity may be
greater when using Fortovase (TM).
& High-level resistance to 3TC develops within 2-4 wks. in partially suppressive regimens;
optimal use is in three-drug antiretroviral combinations that reduce viral load to <500
copies/ mL.
@ The only combination of 2 NRTIs + 1 NNRTI that has been shown to suppress viremia to
undetectable levels in the majority of patients is ZDV + ddI + Nevirapine. This combination
was studied in antiretroviral-naive persons (36).
** ZDV monotherapy may be considered for prophylactic use in pregnant women who have
low viral load and high CD4+ T cell counts to prevent perinatal transmission (see
สConsiderations for Antiretroviral Therapy in the Pregnant HIV-Infected Womanฐ on pages
59-62).
++ This combination of NRTIs is not recommended based on lack of clinical data using the
combination and/or overlapping toxicities.
========================================================================================================
Return to top. Table_7 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 7. Characteristics of nucleoside reverse transcriptase inhibitors (NRTIs)
====================================================================================================================================================
Zidovudine
Generic name (AZT, ZDV) Didanosine (ddI) Zalcitabine (ddC) Stavudine (d4T) Lamivudine (3TC)
Trade name Retrovir Videx HIVID Zerit Epivir
--------------------------------------------------------------------------------------------------------------------------------------------------
Dosing 200 mg t.i.d. or 300 Tablets >60kg: 200 0.75 mg t.i.d. >60 kg: 40 mg b.i.d. 150 mg b.i.d.
recommendations mg b.i.d. or with 3TC mg b.i.d. <60 kg: 30 mg b.i.d. <50 kg: 2 mg/kg b.i.d.
as Combivir (TM), <60 kg: 125 mg b.i.d. or with ZDV as
1 b.i.d. Combivir (TM), 1 b.i.d.
Oral bioavailability 60% Tablet: 40% 85% 86% 86%
Powder: 30%
Serum half-life 1.1 hr. 1.6 hr. 1.2 hr. 1.0 hr. 3-6 hrs.
Intracellular half-life 3 hrs. 25-40 hrs. 3 hrs. 3.5 hrs. 12 hrs.
Elimination Metabolized to AZT Renal excretion 50% Renal excretion 70% Renal excretion 50% Renal excretion
glucuronide (GAZT). unchanged
Renal excretion of
GAZT.
Adverse events Bone marrow Pancreatitis; Peripheral Peripheral (Minimal toxicity)
suppression: anemia Peripheral neuropathy; neuropathy
and/or neutropenia. neuropathy; Nausea; Stomatitis
Subjective Diarrhea
complaints: GI
intolerance,
headache, insomnia,
asthenia.
--------------------------------------------------------------------------------------------------------------------------------------------------
====================================================================================================================================================
Return to top. Table_8 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 8. Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
================================================================================================
Generic name Nevirapine Delavirdine
Trade name Viramune Rescriptor
----------------------------------------------------------------------------------------------
Form 200 mg tabs 100 mg tabs
Dosing recommendations 200 mg po q.d. x 14 days, 400 mg po t.i.d. (four 100 mg
then 200 mg po b.i.d. tabs in >=3 oz. water to produce
slurry)
Oral bioavailability 90% 85%
Serum half-life 25-30 hrs. 5.8 hrs.
Elimination Metabolized by cytochrome Metabolized by cytochrome
p450; 80% excreted in urine p450; 51% excreted in urine
(glucuronidated metabolites, (<5% unchanged); 44% in feces
<5% unchanged); 10% in feces
Drug interactions Induces cytochrome p450 Inhibits cytochrome p450
enzymes enzymes
-- The following drugs have -- Not recommended for
suspected interactions that concurrent use: terfenadine,
require careful monitoring if astemizole, alprazolam,
co-administered with midazolam, cisapride,
nevirapine: rifampin, rifabutin, rifampin,
rifabutin, oral triazolam, ergot derivatives,
contraceptives, protease amphetamines, nifedipine,
inhibitors, triazolam and anticonvulsants (phenytoin,
midazolam. carbamazepine,
phenobarbitol).
-- Delavirdine increases levels
of clarithromycin, dapsone,
quinidine, warfarin,
indinavir, saquinavir.
-- Antacids and didanosine:
separate administration by
>=1 hr.
Adverse events Rash; increased transaminase Rash; headaches
levels; hepatitis
----------------------------------------------------------------------------------------------
================================================================================================
Return to top. Table_9 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 9. Characteristics of protease inhibitors (PIs)
===================================================================================================================================================================
Generic name Indinavir Ritonavir Saquinavir Nelfinavir
-------------------------------------------------
Trade name Crixivan Norvir Invirase (TM) Fortovase (TM) Viracept
-----------------------------------------------------------------------------------------------------------------------------------------------------------------
Form 200-, 400-mg caps 100-mg caps 200-mg caps 200-mg caps 250-mg tablets
600 mg/7.5 mL po 50-mg/g oral powder
solution
Dosing 800 mg q8h 600 mg q12h * 600 mg t.i.d. * 1,200 mg t.i.d. 750 mg t.i.d.
recommendations Take 1 hr. before or 2 Take with food if Take with large meal. Take with large meal. Take with food (meal
hrs. after meals; may possible. or light snack).
take with skim milk
or low-fat meal.
Oral bioavailability 65% (Not determined) hard-gel capsule: soft-gel capsule 20%-80%
4%, erratic (not determined)
Serum half-life 1.5-2 hrs. 3-5 hrs. 1-2 hrs. 1-2 hrs. 3.5-5 hrs.
Route of metabolism P450 cytochrome 3A4 P450 cytochrome P450 cytochrome 3A4 P450 cytochrome 3A4 P450 cytochrome 3A4
3A4>2D6
Storage Room temperature Refrigerate capsules; Room temperature Refrigerate or store Room temperature
refrigeration for oral at room temperature
solution is preferred (up to 3 mos.).
but not required if
used within 30 days.
Adverse effects Nephrolithiasis. GI intolerance, GI intolerance, GI intolerance, Diarrhea.
GI intolerance, nausea, vomiting, nausea and diarrhea. nausea, diarrhea, Hyperglycemia. (@)
nausea. diarrhea. Headache. abdominal pain and
Lab: increased Paresthesias Elevated dyspepsia.
indirect bilirubinemia (circumoral and transaminase Headache.
(inconsequential). extremities). enzymes. Elevated
Miscellaneous: Hepatitis. Hyperglycemia. (@) transaminase
headache, asthenia, Asthenia. enzymes.
blurred vision, Taste perversion. Hyperglycemia. (@)
dizziness, rash, Lab: Triglycerides
metallic taste, increase >200%,
thrombocytopenia. transaminase
Hyperglycemia. (@) elevation, elevated
CPK and uric acid.
Hyperglycemia. (@)
Drug interactions Inhibits cytochrome Inhibits cytochrome Inhibits cytochrome Inhibits cytochrome Inhibits cytochrome
P450 (less than P450 (potent P450. P450. P450 (less than
ritonavir). inhibitor). Saquinavir levels Saquinavir levels ritonavir).
Contraindicated for Ritonavir increases increased by: increased by: Nelfinavir levels
concurrent use: levels of multiple ritonavir, ritonavir, reduced by rifampin,
terfenadine, drugs that are not ketoconazole, ketoconazole, rifabutin.
astemizole, cisapride, recommended for grapefruit juice, grapefruit juice, Contraindicated for
triazolam, concurrent use+. nelfinavir, delavirdine. nelfinavir, delavirdine. concurrent use:
midazolam, ergot Didanosine: may Saquinavir levels Saquinavir levels triazolam,
alkaloids. cause reduced reduced by: rifampin, reduced by: rifampin, midazolam, ergot
Indinavir levels absorption of both rifabutin, and rifabutin, and alkaloid, terfenadine,
increased by: drugs; should be possibly the possibly the astemizole, cisapride.
ketoconazole&, taken >=2 hours apart. following: following: Nelfinavir decreases
delavirdine. Ritonavir decreases phenobarbital, phenobarbital, levels of ethinyl
Indinavir levels levels of ethinyl phenytoin, phenytoin, estradiol and norethindrone.
reduced by: rifampin, estradiol, dexamethasone and dexamethasone and Nelfinavir increases
rifabutin, grapefruit theophylline, carbamezepine, carbamezepine, levels of rifabutin,
juice, nevirapine. sulfamethoxazole nevirapine. nevirapine. saquinavir, and
Didanosine reduces and zidovudine. Contraindicated for Contraindicated for indinavir.
indinavir absorption Ritonavir increases concurrent use: concurrent use: Not recommended
unless taken >2 hrs levels of terfenadine, terfenadine, for concurrent use:
apart. clarithromycin and astemizole, cisapride, astemizole, cisapride, rifampin.
Not recommended desipramine. ergot alkaloids, ergot alkaloids,
for concurrent use: triazolam and triazolam and
rifampin. midazolam. midazolam.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------
* Dose escalation for ritonavir: Day 1-2: 300 mg b.i.d.; day 3-5: 400 mg b.i.d.; day 6-13: 500 mg b.i.d.; day 14: 600 mg b.i.d. Combination
treatment regimen with saquinavir (400-600 mg po b.i.d.) plus ritonavir (400-600 mg po b.i.d.).
+ Drugs contraindicated for concurrent use with ritonavir: amioderone (Cordonrone), astemizole (Hismanal), bepridil (Vascar), bupropion
(Wellbutin), cisapride (Propulsid), clorazepate (Tranxene), clozapine (Clozaril), diazepam (Valium), encainide (Enkaid), estazolam (ProSom),
flecainide (Tambocor), flurazepam (Dalmane), meperidine (Demerol), midazolam (Versed), piroxicam (Feldene), propoxyphene (Darvon),
propafenone (Rythmol), quinidine, rifabutin, terfenadine (Seldane), triazolam (Halcion), zolpidem (Ambien), ergot alkaloids.
& Decrease indinavir to 600 mg q8h.
@ Cases of new onset hyperglycemia have been reported in association with the use of all Pis (41-43).
===================================================================================================================================================================
Return to top. Table_10 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 10. Drugs that should not be used with protease inhibitors
===================================================================================================================================================
Drugs
------------------------------------------------------------------------------------------------------------------------
Saquinavir
(given as
Invirase (TM) or
Drug category Indinavir Ritonavir* Fortovase (TM)) Nelfinavir Alternatives
-------------------------------------------------------------------------------------------------------------------------------------------------
Analgesics (none) meperidine prioxicam (none) (none) ASA, oxycodon
propoxyphene acetaminophen
Cardiac (none) amioderone encainide (none) (none) limited experience
flecainide propafenone
quinidine
Antimycobacterial rifampin rifabutin + rifampin rifampin For rifabutin (as
rifabutin alternative for MAI
treatment):
clarithromycin,
ethambutol (treatment,
not prophylaxis), or
azithromycin
Ca++ channel blocker (none) bepridil (none) (none) limited experience
Antihistamine astemizole astemizole astemizole astemizole loratadine
terfenadine terfenidine terfenidine terfenidine
GI cisapride cisapride cisapride cisapride limited experience
Antidepressant (none) bupropion (none) (none) fluoxetine, desipramine
Neuroleptic (none) clozapine pimozide (none) (none) limited experience
Psychotropic midazolam clorazepate, diazepam midazolam midazolam temazepam, lorazepam
triazolam estazolam, flurazepam triazolam triazolam
midazolam, triazolam
zolpidem
Ergot alkaloid dihydroergot-amine dihydroergotamine
(vasoconstrictor) (D.H.E. 45), ergotamine & (D.H.E. 45), ergotamine &
(various forms) (various forms)
-------------------------------------------------------------------------------------------------------------------------------------------------
* The contraindicated drugs listed are based on theoretical considerations. Thus, drugs with low therapeutic indices yet with suspected
major metabolic contribution from cytochrome P450 3A, CYP2D6, or unknown pathways are included in this table. Actual interactions
may or may not occur in patients.
+ Reduce rifabutin dose to one fourth of the standard dose.
& This is likely a class effect.
===================================================================================================================================================
Return to top. Table_11 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 11. Drug interactions between protease inhibitors and other drugs; drug interactions requiring dose modifications
==================================================================================================================================================
Indinavir Ritonavir Saquinavir * Nelfinavir
------------------------------------------------------------------------------------------------------------------------------------------------
Fluconazole No dose change No dose change No data No dose change
Ketoconazole and Decrease dose to Increases ketoconazole Increases saquinavir levels No dose change
itraconazole 600 mg q8h >3-fold; dose adjustment 3-fold; no dose change+ .
required.
Rifabutin Reduce rifabutin to one Consider alternative drug Not recommended with Reduce rifabutin to one
half dose: 150 mg q.d. or reduce dose to one either Invirase (TM) or half dose: 150 mg q.d.
fourth of standard dose. Fortovase (TM).
Rifampin Contraindicated Unknown & Not recommended with Contraindicated
either Invirase (TM) or
Fortovase (TM).
Oral contraceptives Modest increase in Ethinyl estradiol levels No data Ethinyl estradiol and
Ortho-Novum levels; no decreased; use alternative norethindrone levels
dose change. or additional contraceptive decreased; use alternative
method. or additional contraceptive
method.
Miscellaneous Grapefruit juice reduces Desipramine increased Grapefruit juice increases
indinavir levels by 26%. 145%: reduce dose; saquinavir levels +.
Theophylline levels
decreased: increase dose.
------------------------------------------------------------------------------------------------------------------------------------------------
* Several drug interaction studies have been completed with saquinavir given as Invirase (TM) or Fortovase (TM) . Results from studies
conducted with Invirase (TM) may not be applicable to Fortovase (TM) .
+ Conducted with Invirase (TM) .
& Rifampin reduces ritonavir 35%. Increased ritonavir dose or use of ritonavir in combination therapy is strongly recommended. The effect
of ritonavir on rifampin is unknown. Used concurrently, increased liver toxicity may occur. Therefore, patients on ritonavir and rifampin
should be monitored closely.
==================================================================================================================================================
Return to top. Table_12 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 12. Drug interactions: protease inhibitors and non-nucleoside reverse transcriptase inhibitors -- effect of drug on
levels/dose
===============================================================================================================================================================
Drug affected Indinavir Ritonavir Saquinavir* Nelfinavir Nevirapine Delavirdine
-------------------------------------------------------------------------------------------------------------------------------------------------------------
Indinavir (IDV) -- No data Levels: IDV no Levels: IDV /\50%; Levels: IDV บบ28% Levels: IDV /\40%
effect; SQV /\4-7x & บบ \/ บบ
บบ NFV /\80% Dose: standard Dose: IDV 600 mg
Dose: no data บบ q8h
Dose: no data
Ritonavir (RTV) No data -- Levels: RTV no Levels: RTV no Levels: RTV บบ11% Levels: RTV /\70%
effect; SQV /\20x +& effect; NFV /\1.5x \/ บบ
บบ บบ Dose: standard Dose: no data
Dose: Invirase (TM) Dose: no data
or Fortovase (TM)
400 mg b.i.d.
+RTV: 400 mg b.i.d.
Saquinavir (SQV) Levels: SQV Levels: SQV -- Levels: SQV Levels: SQV บบ25% + Levels: SQV /\5x +
/\4-7x; IDV no /\20x +& RTV no /\3-5x; NFV /\20%& \/ บบ
บบ บบ บบ บบ Dose: no data Dose: standard
effect & effect Dose: standard for Invirase (TM)
Dose: no data Dose: Invirase (TM) NFV Fortovase (TM) Monitor
or Fortovase (TM) 800 mg t.i.d. transaminase
400 mg b.i.d. levels
+RTV 400 mg b.i.d.
Nelfinavir (NFV) Levels: NFV /\80% Levels: NFV /\1.5x Levels: NFV /\20%; -- Levels: NFV /\10% Levels: NFV /\2x
บบ บบ บบ บบ บบ
IDV /\50% RTV no effect SQV /\3-5x & Dose: standard DLV บบ50%
บบ Dose: no data บบ \/
Dose: no data Dose: standard Dose: standard
NFV Fortovase (TM) (monitor for
800 mg t.i.d. neutropenic
complications)
Nevirapine (NVP) Levels: IDV บบ28% Levels: RTV /\11% Levels: SQV บบ25%+; Levels: NFV /\10% -- Do not use
\/ บบ \/ บบ together
Dose: standard Dose: standard Dose: no data Dose: standard
Delavirdine (DLV) Levels: IDV /\40% Levels: RTV /\70% Levels: SQV i5x + Levels: NFV /\2x Do not use --
บบ บบ Dose: standard บบ together
Dose: IDV 600 q8h Dose: no data for Invirase (TM) DLV บบ50%
Monitor \/
transaminase Dose: standard
levels (monitor for
neutropenic
complications)
-------------------------------------------------------------------------------------------------------------------------------------------------------------
* Several drug interaction studies have been completed with saquinavir given as Invirase (TM) or Fortovase (TM). Results from studies
conducted with Invirase (TM) may not be applicable to Fortovase (TM) .
+ Conducted with Invirase (TM).
& Conducted with Fortovase (TM).
===============================================================================================================================================================
Return to top. Table_13 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. Table 13. Guidelines for changing an antiretroviral regimen for suspected drug failure -------------------------------------------------------------------------------------------- -- Criteria for changing therapy include a suboptimal reduction in plasma viremia after initiation of therapy, reappearance of viremia after suppression to undetectable, substantial increases in plasma viremia from the nadir of suppression, and declining CD4 + T cell numbers. Refer to the more extensive discussion of these criteria in "Criteria for Changing Therapy" on pages 53-54. -- When the decision to change therapy is based on viral load determination, it is preferable to confirm with a second viral load test. -- Distinguish between the need to change a regimen because of drug intolerance or inability to comply with the regimen versus failure to achieve the goal of sustained viral suppression; single agents can be changed or dose reduced in the event of drug intolerance. -- In general, do not change a single drug or add a single drug to a failing regimen; it is important to use at least two new drugs and preferably to use an entirely new regimen with at least three new drugs. -- Many patients have limited options for new regimens of desired potency; in some of these cases, it is rational to continue the prior regimen if partial viral suppression was achieved. -- In some cases, regimens identified as suboptimal for initial therapy are rational due to limitations imposed by toxicity, intolerance, or nonadherence. This especially applies in late-stage disease. For patients with no rational alternative options who have virologic failure with return of viral load to baseline (pretreatment levels) and a declining CD4+ T cell count, discontinuation of antiretroviral therapy should be considered. -- Experience is limited with regimens using combinations of two protease inhibitors or combinations of protease inhibitors with nevirapine or delavirdine; for patients with limited options due to drug intolerance or suspected resistance, these regimens provide possible alternative treatment options. -- There is limited information about the value of restarting a drug that the patient has previously received. The experience with zidovudine is that resistant strains are often replaced with "wild-type" zidovudine sensitive strains when zidovudine treatment is stopped, but resistance recurs rapidly if zidovudine is restarted. Although preliminary evidence indicates that this occurs with indinavir, it is not known if similar problems apply to other nucleoside analogues, protease inhibitors, or NNRTIs, but a conservative stance is that they probably do. -- Avoid changing from ritonavir to indinavir or vice versa for drug failure, because high-level cross-resistance is likely. -- Avoid changing from nevirapine to delavirdine or vice versa for drug failure, because high-level cross-resistance is likely. -- The decision to change therapy and the choice of a new regimen require that the clinician have considerable expertise in the care of persons living with HIV infection. Physicians who are less experienced in the care of persons with HIV infection are strongly encouraged to obtain assistance through consultation with or referral to a clinician who has considerable expertise in the care of HIV-infected patients. -------------------------------------------------------------------------------------------- Return to top. Table_14 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 14. Possible regimens for patients who have failed antiretroviral therapy: a
work in progress*
======================================================================================================
Prior regimen New regimen (not listed in priority order)
------------------------------------------------------------------
2 NRTIs + 2 new NRTIs +
Nelfinavir (NFV) RTV; or IDV; or SQV + RTV; or NNRTI+ +
RTV; or NNRTI + IDV&
Ritonavir (RTV) SQV + RTV&; NFV + NNRTI; or NFV + SQV
Indinavir (IDV) SQV + RTV; NFV + NNRTI; or NFV + SQV
Saquinavir (SQV) RTV + SQV; or NNRTI + IDV
2 NRTIs + NNRTI 2 new NRTIs + a protease inhibitor
2 NRTIs 2 new NRTIs + a protease inhibitor
2 new NRTIs + RTV + SQV
1 new NRTI + 1 NNRTI + a protease inhibit
2 protease inhibitors + NNRTI
1 NRTI 2 new NRTIs + a protease inhibitor
2 new NRTIs + NNRTI
1 new NRTI + 1 NNRTI + a protease inhibitor
------------------------------------------------------------------
* These alternative regimens have not been proven to be clinically effective and were arrived
at through discussion by the panel of theoretically possible alternative treatments and the
elimination of those alternatives with evidence of being ineffective. Clinical trials in this area
are urgently needed.
+ Of the two available NNRTIs, clinical trials support a preference for nevirapine over delavirdine
based on results of viral load assays. These two agents have opposite effects on the CYP450
pathway, and this must be considered in combining these drugs with other agents.
& There are some clinical trials that have yielded viral burden data to support this recommen-
dation.
======================================================================================================
Return to top. Table_15 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 15. Acute retroviral syndrome: associated signs and symptoms and expected
frequency *
-------------------------------------------------------------------------------
-- Fever (96%)
-- Lymphadenopathy (74%)
-- Pharyngitis (70%)
-- Rash (70%)
Erythematous maculopapular with lesions on face and trunk and sometimes
extremities, including palms and soles
Mucocutaneous ulceration involving mouth, esophagus, or genitals
-- Myalgia or arthralgia (54%)
-- Diarrhea (32%)
-- Headache (32%)
-- Nausea and vomiting (27%)
-- Hepatosplenomegaly (14%)
-- Thrush (12%)
-- Weight Loss
-- Neurologic symptoms (12%)
Meningoencephalitis or aseptic meningitis
Peripheral neuropathy or radiculopathy
Facial palsy
Guillain-Barre syndrome
Brachial neuritis
Cognitive impairment or psychosis
-------------------------------------------------------------------------------
* Adapted from reference 19.
Return to top. Table_16 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 16. Preclinical and clinical data relevant to use of antiretrovirals during pregnancy
========================================================================================================
FDA-defined Placental passage Long-term animal
Antiretroviral pregnancy {Newborn: carcinogenicity
drug category* maternal drug} studies Rodent teratogen
------------------------------------------------------------------------------------------------------
Zidovudine+ C Yes (human) {0.85} Positive (rodent, Positive (near lethal
vaginal tumors) dose)
Zalcitabine C Yes (rhesus) Positive (rodent, Positive
{0.30-0.50} thymic lymphomas) (hydrocephalus at
high dose)
Didanosine B Yes (human) {0.5} Negative (no Negative
tumors, lifetime
rodent study)
Stavudine C Yes (rhesus) {0.76} Not completed Negative (but
sternal bone
calcium decreases)
Lamivudine C Yes (human){~1.0} Negative (no Negative
tumors, lifetime
rodent study)
Saquinavir B Unknown Not completed Negative
Indinavir C Yes (rats) Not completed Negative (but extra
(สSignificantฐ in ribs in rats)
rats; low in rabbits)
Ritonavir B Yes (rats) {mid-term Not completed Negative (but
fetus, 1.15; cryptorchidism in
late-term fetus, rats)& |