 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Report of the NIH Panel to Define Principles of Therapy of HIV InfectionPreface The past 2 years have witnessed remarkable advances in the development of antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection, as well as measurement of HIV plasma RNA (viral load) to guide the use of antiretroviral drugs. The use of ART, in conjunction with the prevention of specific HIV- related opportunistic infections (OIs), has been associated with dramatic decreases in the incidence of OIs, hospitalizations, and deaths among HIV- infected persons. Advances in this field have been so rapid, however, that keeping up with them has posed a formidable challenge to health- care providers and to patients, as well as to institutions charged with the responsibility of paying for these therapies. Thus, the Office of AIDS Research, the National Institutes of Health, and the Department of Health and Human Services, in collaboration with the Henry J. Kaiser Foundation, have assumed a leadership role in formulating the scientific principles (NIH Panel) and developing the guidelines (DHHS/ Kaiser Panel) for the use of antiretroviral drugs that are presented in this report. CDC staff participated in these efforts, and CDC and MMWR are pleased to be able to provide this information as a service to its readers. This report is targeted primarily to providers who care for HIV-infected persons, but it also is intended for patients, payors, pharmacists, and public health officials. The report comprises two articles. The first article, Report of the NIH Panel To Define Principles of Therapy of HIV Infection, provides the basis for the use of antiretroviral drugs, and the second article, Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents, provides specific recommendations regarding when to start, how to monitor, and when to change therapy, as well as specific combinations of drugs that should be considered. Both articles provide cross-references to each other so readers can locate related information. Tables and figures are included in the Appendices section that follows each article. Although the principles are unlikely to change in the near future, the guidelines will change substantially as new information and new drugs become available. Copies of this document and all updates are available from the CDC National AIDS Clearinghouse (1-800-458-5231) and are posted on the Clearinghouse World-Wide Web site (http://www.cdcnac.org). In addition, copies and updates also are available from the HIV/AIDS Treatment Information Service (1-800-448-0440; Fax 301-519-6616; TTY 1-800-243-7012) and on the ATIS World-Wide Web site (http://www.hivatis.org). Readers should consult these web sites regularly for updates in the guidelines. Report of the NIH Panel To Define Principles of Therapy of HIV Infection Panel Members Charles Carpenter, M.D. Julia Hidalgo, S.C.D. Chair Center for AIDS Services Planning Brown University and Development The Miriam Hospital Baltimore, MD Providence, RI Harold Jaffe, M.D. Mark Feinberg, M.D., Ph.D. Centers for Disease Control Executive Secretary and Prevention National Institutes of Health Atlanta, GA Bethesda, MD Dan Landers, M.D. Wade Aubry, M.D. Magee Women's Hospital Blue Cross/ Blue Shield Association Pittsburgh, PA San Francisco, CA Henry Masur, M.D. Dawn Averitt National Institutes of Health Women's Information Service Bethesda, MD and Exchange (WISE) Atlanta, GA Philip Pizzo, M.D. Children's Hospital/Harvard Medical John Coffin, Ph.D. School Tufts University School of Medicine Boston, MA Boston, MA Douglas Richman, M.D. David Cooper, M.D. University of California, San Diego National Center for HIV Epidemiology La Jolla, CA and Clinical Research Sydney, NSW, Australia Michael Saag, M.D. University of Alabama, Birmingham Stephen Follansbee, M.D. Birmingham, AL Davies Medical Center San Francisco, CA Robert Schooley, M.D. University of Colorado Health Peggy Hamburg, M.D. Sciences Center New York City Department of Health Denver, CO New York, NY Valerie Stone, M.D., M.P.H. Mark Harrington Brown University School of Medicine Treatment Action Group Pawtucket, RI New York, NY Bruce Walker, M.D. Melanie Thompson, M.D. Harvard Medical School AIDS Research Consortium of Atlanta Boston, MA Atlanta, GA Patrick Yeni, M.D. Didier Trono, M.D. X. Bichat Medical School The Salk Institute for Biological Paris, France Studies La Jolla, CA Stefano Vella, M.D. Instituto Superiore di Sanita Laboratory of Virology Rome, Italy The material in this report was prepared for publication by: Mark B. Feinberg, M.D., Ph.D. Office of AIDS Research National Institutes of Health in collaboration with Jonathan E. Kaplan, M.D. Division of AIDS, STD, and TB Laboratory Research National Center for Infectious Diseases and Division of HIV/AIDS Prevention Surveillance, and Epidemiology National Center for HIV, STD, and TB Prevention Report of the NIH Panel To Define Principles of Therapy of HIV Infection * Summary Recent research advances have afforded substantially improved understanding of the biology of human immunodeficiency virus (HIV) infection and the pathogenesis of the acquired immunodeficiency syndrome (AIDS). With the advent of sensitive tools for monitoring HIV replication in infected persons, the risk of disease progression and death can be assessed accurately and the efficacy of anti-HIV therapies can be determined directly. Furthermore, when used appropriately, combinations of newly available, potent antiviral therapies can effect prolonged suppression of detectable levels of HIV replication and circumvent the inherent tendency of HIV to generate drug-resistant viral variants. However, as antiretroviral therapy for HIV infection has become increasingly effective, it has also become increasingly complex. Familiarity with recent research advances is needed to ensure that newly available therapies are used in ways that most effectively improve the health and prolong the lives of HIV-infected persons. To enable practitioners and HIV-infected persons to best use rapidly accumulating new information about HIV disease pathogenesis and treatment, the Office of AIDS Research of the National Institutes of Health sponsored the NIH Panel to Define Principles of Therapy of HIV Infection. This Panel was asked to define essential scientific principles that should be used to guide the most effective use of antiretroviral therapies and viral load testing in clinical practice. Based on detailed consideration of the most current data, the Panel delineated eleven principles that address issues of fundamental importance for the treatment of HIV infection. These principles provide the scientific basis for the specific treatment recommendations made by the Panel on Clinical Practices for the Treatment of HIV Infection sponsored by the Department of Health and Human Services and the Henry J. Kaiser Family Foundation. The reports of both of these panels are provided in this publication. Together, they summarize new dta and provide both the scientific basis and specific guidelines for the treatment of HIV-infected persons. This information will be of interest to health-care providers, HIV-infected persons, HIV/AIDS educators, public health educators, public health authorities, and all organizations that fund medical care of HIV-infected persons. INTRODUCTION The past 2 years have brought major advances in both basic and clinical research on acquired immunodeficiency syndrome (AIDS). The availability of more numerous and more potent drugs to inhibit human immunodeficiency virus (HIV) replication has made it possible to design therapeutic strategies involving combinations of antiretroviral drugs that accomplish prolonged and near complete suppression of detectable HIV replication in many HIV-infected persons. In addition, more sensitive and reliable measurements of plasma viral load have been demonstrated to be powerful predictors of a person's risk for progression to AIDS and time to death. They have also been demonstrated to reliably assess the antiviral activity of therapeutic agents. It is now critical that these scientific advances be translated into information that practitioners and their patients can utilize in making decisions about using the new therapies and monitoring tools to achieve the greatest, most durable clinical benefits. Such information will allow physicians to tailor more effective treatments for their patients and to more closely monitor patients' responses to specific antiretroviral regimens. A two-track process was initiated to address this pressing need. The Office of AIDS Research of the National Institutes of Health (NIH) sponsored the NIH Panel To Define Principles of Therapy of HIV Infection. This Panel was asked to delineate the scientific principles, based on its understanding of the biology and pathogenesis of HIV infection and disease, that should be used to guide the most effective use of antiretroviral therapy and viral load testing in clinical practice. The Department of Health and Human Services (HHS) and the Henry J. Kaiser Family Foundation sponsored the Panel on Clinical Practices for the Treatment of HIV Infection. The HHS Panel was charged with developing recommendations, based on the scientific principles, for the clinical use of antiretroviral drugs and laboratory monitoring methods in the treatment of HIV-infected persons. Both documents -- the Report of the NIH Panel To Define Principles of Therapy for HIV Infection, developed by the NIH Panel, and the Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents, developed by the HHS Panel -- are provided in this report. Together, these two documents summarize new data and provide both the scientific basis and specific guidelines for the treatment of HIV-infected persons. The goal of this report is to assist clinicians and patients in making informed decisions about treatment options so that a) effective antiretroviral therapy is introduced before extensive immune system damage has occurred; b) viral load monitoring is used as an essential tool in determining an HIV-infected person's risk for disease progression and response to antiretroviral therapy; c) combinations of antiretroviral drugs are used to suppress HIV replication to below the limits of detection of sensitive viral load assays; and d) patient adherence to the complicated regimens of combination antiretroviral therapy that are currently required to achieve durable suppression of HIV replication is encouraged by patient-provider relationships that provide education and support concerning the goals, strategies, and requirements of antiretroviral therapy. The NIH Panel included clinicians, basic and clinical researchers, public health officials, and community representatives. As part of its effort to accumulate the most current data, the Panel held a 2-day public meeting to hear presentations by clinicians and scientists in the areas of HIV pathogenesis and treatment, specifically addressing the following topics: the relationship between virus replication and disease progression; the relative ability of available strategies of antiviral therapy to minimize HIV replication for prolonged periods of time; the relationship between the emergence of drug resistance and treatment failures; the relative ability of available strategies of antiviral therapy to delay or prevent the emergence of drug-resistant HIV variants; and the relationship between drug-induced changes in virus load and improved clinical outcomes and prolonged survival. Summary of the Principles of Therapy of HIV Infection
scientific principles were derived from three primary sources: recent basic insights into the life cycle of HIV, studies of the extent and consequences of HIV replication in infected persons, and clinical trials of anti-HIV drugs. In certain instances, the Panel based the principles and associated corollaries on clinical studies conducted in relatively small numbers of patients for fairly short periods of time. After carefully evaluating data from these studies, the Panel concluded that the results of several important contemporary studies have been consistent in their validation of recent models of HIV pathogenesis. The Panel believes that new antiretroviral drugs and treatment strategies, if used correctly, can substantially benefit HIV-infected persons. However, as the understanding of HIV disease has improved and the number of available beneficial therapies has increased, clinical care of HIV-infected patients has become much more complex. Therapeutic success increasingly depends on a thorough understanding of the pathogenesis of HIV disease and on familiarity with when and how to use the more numerous and more effective drugs available to treat HIV infection. The Panel is concerned that even these new potent antiretroviral therapies will be of little clinical utility for treated patients unless they are used correctly and that, used incorrectly, they may even compromise the potential to obtain long-term benefit from other antiretroviral therapies in the future. The principles and conclusions discussed in this report have been developed and made available now so that practitioners and patients can make treatment decisions based on the most current research results. Undoubtedly, insights into the pathogenesis of HIV disease will continue to accumulate rapidly, providing new targets for the development of additional antiretroviral drugs and even more effective treatment strategies. Thus, the Panel expects that these principles will require modification and elaboration as new information is acquired. SCIENTIFIC PRINCIPLES Principle 1. Ongoing HIV replication leads to immune system damage and progression to AIDS. HIV infection is always harmful, and true long-term survival free of clinically significant immune dysfunction is unusual. Active replication of HIV is the cause of progressive immune system damage in infected persons (1-10). In the absence of effective inhibition of HIV replication by antiretroviral therapy, nearly all infected persons will suffer progressive deterioration of immune function resulting in their susceptibility to opportunistic infections (OIs), malignancies, neurologic diseases, and wasting, ultimately leading to death (11,12). For adults who live in developed countries, the average time of progression to AIDS after initial infection is approximately 10-11 years in the absence of antiretroviral therapy or with older regimens of nucleoside analog (e.g., zidovudine {ZDV}) monotherapy (11). Some persons develop AIDS within 5 years of infection (20%), whereas others (less than 5%) have sustained long-term (greater than 10 years) asymptomatic HIV infection without decline of CD4+ T cell counts to less than 500cells/mm3. Only approximately 2% or less of HIV-infected persons seem to be able to contain HIV replication to extremely low levels and maintain stable CD4+ T cell counts within the normal range for lengthy periods (greater than 12 years), and many of these persons display laboratory evidence of immune system damage (12). Thus, HIV infection is unusual among human virus infections in causing disease in such a large proportion of infected persons. Although a very small number of HIV-infected persons do not demonstrate progressive HIV disease in the absence of antiretroviral therapy, there is no definitive way to prospectively identify these persons. Therefore, all persons who have HIV infection must be considered at risk for progressive disease. The goals of treatment for HIV infection should be to maintain immune function in as near a normal state as possible, prevent disease progression, prolong survival, and preserve quality of life by effectively suppressing HIV replication. For these goals to be accomplished, therapy should be initiated, whenever possible, before extensive immune system damage has occurred. Principle 2. Plasma HIV RNA levels indicate the magnitude of HIV replication and its associated rate of CD4+ T cell destruction, whereas CD4+ T cell counts indicate the extent of HIV-induced immune damage already suffered. Regular, periodic measurement of plasma HIV RNA levels and CD4+ T cell counts is necessary to determine the risk for disease progression in an HIV-infected person and to determine when to initiate or modify antiretroviral treatment regimens. The rate of progression of HIV disease is predicted by the magnitude of active HIV replication (reflected by so-called viral load) taking place in an infected person (5-10,13-18). Measurement of viral load through the use of quantitative plasma HIV RNA assays permits assessment of the relative risk for disease progression and time to death (5-10,13-18). Plasma HIV RNA measurements also permit assessment of the efficacy of antiretroviral therapies in individual patients (1,2,13,19-25). It is expert opinion that these measurements are necessary components of treatment strategies designed to use antiretroviral drugs most effectively. The extent of immune system damage that has already occurred in an HIV-infected person is indicated by the CD4+ T cell count (11,26-29), which permits assessment of the risk for developing specific OIs and other sequelae of HIV infection. When used in concert with viral load determinations, assessment of CD4+ T cell number enhances the accuracy with which the risk for disease progression and death can be predicted (27). Issues specific for the laboratory assessment of plasma HIV RNA and CD4+ T cell levels in HIV-infected infants and young children are discussed in Principle 9 (14-18,25,30). Important specific considerations regarding laboratory evaluations and HIV-infected persons include the following:
Principle 3. As rates of disease progression differ among HIV-infected persons, treatment decisions should be individualized by level of risk indicated by plasma HIV RNA levels and CD4+ T cell counts. Decisions regarding when to initiate antiretroviral therapy in an HIV-infected person should be based on the risk for disease progression and degree of immunodeficiency. Initiation of antiretroviral therapy before the onset of immunologic and virologic evidence of disease progression is expected to have the greatest and most durable beneficial impact on preserving the health of HIV-infected persons. When specific viral load or CD4+ T cell levels at which therapy should be initiated are considered, it is important to recognize that the risk for disease progression is a continuous rather than discrete function (5,6,10,27). There is no known absolute threshold of HIV replication below which disease progression will not eventually occur. At present, recommendations for initiation of therapy must be based on the fact that the types and numbers of available antiretroviral drugs are limited. When more numerous, more effective, better tolerated, and more conveniently dosed drugs become available, it is likely that indications for initiation of therapy will change accordingly. Specific considerations regarding treatment include the following:
Principle 4. The use of potent combination antiretroviral therapy to suppress HIV replication to below the levels of detection of sensitive plasma HIV RNA assays limits the potential for selection of antiretroviral-resistant HIV variants, the major factor limiting the ability of antiretroviral drugs to inhibit virus replication and delay disease progression. Therefore, maximum achievable suppression of HIV replication should be the goal of therapy. Studies of the biology and pathogenesis of HIV infection have provided the basis for using antiretroviral drugs in ways that yield the most profound and durable suppression of HIV replication. The inherent ability of HIV to develop drug resistance represents the major obstacle to the long-term efficacy of antiretroviral therapy (21). However, recent clinical evidence indicates that the development of drug resistance can be delayed, and perhaps even prevented, by the rational use of combinations of drugs that include newly available, potent agents to suppress HIV replication to levels that cannot be detected by sensitive assays of plasma HIV RNA (23,38-40). Cessation of detectable HIV replication decreases the opportunity for accumulation of mutations that may give rise to drug-resistant viral variants. Furthermore, the extent and duration of inhibition of HIV replication by antiretroviral therapy predicts the magnitude of clinical benefit derived from treatment (9,13,23-25). The potential toxicities of therapy, as well as the patient's quality of life and ability to adhere to a complex antiretroviral drug regimen, should be balanced with the anticipated clinical benefit of maximal suppression of HIV replication and the anticipated risks of less complete suppression. Specific considerations regarding treatment include the following:
Principle 5. The most effective means to accomplish durable suppression of HIV replication is the simultaneous initiation of combinations of effective anti-HIV drugs with which the patient has not been previously treated and that are not cross-resistant with antiretroviral agents with which the patient has been previously treated. Several issues should be considered regarding the combination of antiretroviral drugs to be used in the treatment of an HIV-infected patient. The efficacy of a given regimen of combination antiretroviral therapy is not simply a function of the number of drugs used. The most effective antiretroviral drugs possess high potency, favorable pharmacologic properties, and require that HIV acquire multiple mutations in the relevant HIV target gene before high-level drug resistance is realized. In addition, drug-resistant HIV variants selected for by treatment with certain antiretroviral drugs may display diminished ability to replicate (decreased "fitness") in infected persons (21). Drugs used in combination should show evidence of additivity or synergy of antiretroviral activity, should lack antagonistic pharmacokinetic or antiretroviral properties, and should possess nonoverlapping toxicities. Ideally, the chosen drugs will display molecular interactions that increase the potency of antiretroviral therapy or delay the emergence of antiretroviral drug resistance. If multiple options are available for combination therapy, specific antiretroviral drugs should be employed so that future therapeutic options are preserved if the initial choice of therapy fails to achieve its desired result. Whenever possible, therapy should be initiated or modified with a rational combination of antiretroviral drugs, a predefined target for the degree of suppression of HIV replication desired, and a predefined alternative antiretroviral regimen to be used should the target goal not be reached. Specific considerations regarding treatment include the following:
Principle 6. Each of the antiretroviral drugs used in combination therapy regimens should always be used according to optimum schedules and dosages. The use of combinations of potent antiretroviral drugs to exert constant, maximal suppression of HIV replication provides the best approach to circumvent the inherent tendency of HIV to generate drug-resistant variants. Specific considerations regarding treatment include the following:
Principle 7. The available effective drugs are limited in number and mechanism of action, and cross-resistance between specific drugs has been documented. Therefore, any change in antiretroviral therapy increases future therapeutic constraints. Decisions to alter therapy will rely heavily on consideration of clinical issues and on the number of available alternative antiretroviral agents. Every decision made to alter therapy may limit future treatment options. Thus, available agents should not be abandoned prematurely. It is not known definitively whether the pathogenic consequences of a measurable level of HIV replication while on therapy are equivalent to those of an equivalent level in an untreated person; however, preliminary data suggest that this is the case. Thus, the level at which HIV replication continues while on an antiretroviral drug regimen that has failed to suppress plasma HIV RNA to below detectable levels should be considered as an indication of the urgency with which an alteration in therapy should be pursued. Specific considerations regarding treatment include the following:
Principle 8. Women should receive optimal antiretroviral therapy regardless of pregnancy status. The use of antiretroviral treatment in HIV-infected pregnant women raises important, unique concerns (64). HIV counseling and the offer of HIV testing to pregnant women have been universally recommended in the United States and are now mandatory in some states. A greater awareness of issues surrounding HIV infection in pregnant women has resulted in an increased number of women whose initial diagnosis of HIV infection is made during pregnancy. In this circumstance, or when women already aware of their HIV infection become pregnant, treatment decisions should be based on the current and future health of the mother, as well as on preventing perinatal transmission and ensuring the health of the fetus and neonate. Care of the HIV-infected pregnant woman should involve a collaboration between the HIV specialist caring for the woman when she is not pregnant, her obstetrician, and the woman herself. Treatment recommendations for HIV-infected pregnant women are based on the belief that therapies of known benefit to women should not be withheld during pregnancy unless there are known adverse effects on the mother, fetus, or infant that outweigh the potential benefit to the woman (64). There are two separate but interconnected issues regarding antiretroviral treatment during pregnancy: a) use of antiretroviral therapy for maternal health indications and b) use of antiretroviral drugs for reducing the risk of perinatal HIV transmission. Although zidovudine monotherapy substantially reduces the risk of perinatal HIV transmission, appropriate combinations of antiretroviral drugs should be administered if indicated on the basis of the mother's health. In general, pregnancy should not compromise optimal HIV therapy for the mother. Specific considerations regarding treatment of pregnant women include the following:
Principle 9. The same principles of antiretroviral therapy apply to HIV-infected children, adolescents, and adults, although the treatment of HIV-infected children involves unique pharmacologic, virologic, and immunologic considerations. Most of the data that support the principles of antiretroviral therapy outlined in this document have been generated in studies of HIV-infected adults. Adolescents infected with HIV sexually or through drug use appear to follow a clinical course similar to adults, and recommendations for antiretroviral therapy for these persons are the same as for adults (see Guidelines). However, although fewer data are available concerning treatment of HIV infection in younger persons, it is unlikely that the fundamental principles of HIV disease differ for HIV-infected children. Furthermore, the data that are available from studies of HIV-infected infants and children indicate that the same fundamental virologic principles apply, and optimal treatment approaches are also likely to be similar (14-18,25). Therefore, HIV-infected children, as previously described for HIV-infected adults, should be treated with effective combinations of antiretroviral drugs with the intent of accomplishing durable suppression of detectable levels of HIV replication. Unfortunately, not all of the antiretroviral drugs that have demonstrated efficacy in combination therapy regimens in adults are available in formulations (e.g., palatable liquid formulations) for infants and young children (particularly for those aged less than 2 years). In addition, pharmacokinetic and pharmacodynamic studies of some antiretroviral agents have yet to be completed in children. Thus, effective antiretroviral therapies should be studied in children and age-specific pharmacologic properties of these therapies should be defined. Antiretroviral drugs selected to treat HIV-infected children should be used only if their pharmacologic properties have been defined in the relevant age group of the patient. Use of antiretroviral drugs before these properties have been defined may result in undesirable toxicities without virologic or clinical benefit. Identification of HIV-infected infants soon after delivery or during the first few weeks following their birth provides opportunities for treatment of primary HIV infection and, perhaps, for facilitating the most effective treatment responses (16-18,66). Thus, identification of HIV-infected women through voluntary testing, provision of antiretroviral therapy to the mother and infant to decrease the risk of maternal-infant transmission, and careful screening of infants born to HIV-infected mothers for evidence of HIV infection will provide an effective strategy to ameliorate the risk and consequences of perinatal HIV infection. The specific HIV RNA and CD4+ T cell criteria used for making decisions about when to initiate therapy in infected adults do not apply directly to newborns, infants, and young children (14-18). As with adults, higher levels of plasma HIV RNA are associated with a greater risk of disease progression and death in infants and young children (14-18). However, absolute levels of plasma HIV RNA observed during the first years of life in HIV-infected children are frequently higher than those found in adults infected for similar lengths of time, and establishment of a post-primary-infection set-point takes substantially longer in infected children (15-18). The increased susceptibility of children to OIs, particularly Pneumocystis carinii pneumonia (PCP), at higher CD4+ T cell counts than HIV-infected adults (30) further indicates that the CD4+ T cell criteria suggested as guides for initiation of antiretroviral therapy in HIV-infected adults are not appropriate to guide therapeutic decisions for infected children. In all, the need for and potential benefits of early institution of effective antiretroviral therapy are likely to be even greater in children than adults, suggesting that most, if not all, HIV-infected children should be treated with effective combination antiretroviral therapies. Principle 10. Persons identified during acute primary HIV infection should be treated with combination antiretroviral therapy to suppress virus replication to levels below the limit of detection of sensitive plasma HIV RNA assays. Studies of HIV pathogenesis provide theoretical support for the benefits of antiretroviral therapy for persons diagnosed with primary HIV infection, and data that are accumulating from small-scale clinical studies are consistent with these predictions (49,66-73). Results from studies suggest that antiretroviral therapy during primary infection may preserve immune system function by blunting the high level of HIV replication and immune system damage occurring during this period and potentially reducing set-point levels of HIV replication, thereby favorably altering the subsequent clinical course of the infection; however, this outcome has yet to be formally demonstrated (51,73). It has been further suggested that the best opportunity to eradicate HIV infection might be provided by the initiation of potent combination antiretroviral therapy during primary infection (51). The Panel believes that, although the long-term benefits of effective combination antiretroviral therapy of primary infection are not known, it is a critical topic of investigation. Therefore, enrollment of newly diagnosed patients in clinical trials should be encouraged to help in defining the optimal approach to treatment of primary infection. When this is neither feasible nor desired, the Panel believes that combination antiretroviral therapy with the goal of suppression of HIV replication to undetectable levels should be pursued. The Panel believes that suppressive antiretroviral therapy for acute primary HIV infection should be continued indefinitely until clinical trials provide data to establish the appropriate duration of therapy. Principle 11. HIV-infected persons, even those whose viral loads are below detectable limits. Therefore, they should be considered infectious. Therefore, they should be counseled to avoid sexual and drug-use behaviors that are associated with either transmission or acquisition of HIV and other infectious pathogens. No data are available concerning the ability of HIV-infected persons who have antiretroviral therapy-induced suppression of HIV replication to undetectable levels (assessed by plasma HIV RNA assays) to transmit the infection to others. Similarly, their ability to acquire a multiply resistant HIV variant from another person remains a possibility. HIV-infected persons who are receiving antiretroviral therapy continue to be able to transmit serious infectious diseases to others (e.g., hepatitis B and C and sexually transmitted infections, such as herpes simplex virus, human papillomavirus syphilis, gonorrhea, chancroid, and chlamydia) and are themselves at risk for infection with these pathogens, as well as others that carry serious consequences for immunosuppressed persons, including cytomegalovirus and human herpes virus 8 (also known as KSHV). Therefore, all HIV-infected persons, including those receiving effective antiretroviral therapies, should be counseled to avoid behaviors associated with the transmission of HIV and other infectious agents. Continued reinforcement that all HIV-infected persons adhere to safe-sex practices is important. If an HIV-infected injecting-drug user is unable or unwilling to refrain from using injection drugs, that person should be counseled to avoid sharing injection equipment with others and to use sterile, disposable needles and syringes for each injection. SCIENTIFIC BACKGROUND HIV Infection Leads to Progressive Immune System Damage in Nearly All Infected Persons Early efforts to synthesize a coherent model of the pathogenic consequences of HIV infection were based on the presumption that few cells in infected persons harbor or produce HIV and that virus replication is restricted during the period of clinical latency. However, early virus detection methods were insensitive, and newer, more sensitive tests have demonstrated that virus replication is active throughout the course of the infection and proceeds at levels far higher than previously imagined. HIV replication has been directly linked to the process of T cell destruction and depletion. In addition, ongoing HIV replication in the face of an active but incompletely effective host antiviral immune response is probably responsible for the secondary manifestations of HIV disease, including wasting and dementia. Beginning with the first cycles of virus replication within the newly infected host, HIV infection results in the progressive destruction of the population of CD4+ T cells that serve essential roles in the generation and maintenance of host immune responses (1-10). The target cell preference for HIV infection and depletion is determined by the identity of the cell surface molecule, CD4, that is recognized by the HIV envelope (Env) glycoprotein as the virus binds to and enters host cells to initiate the virus replication cycle (74). Additional cell surface molecules that normally function as receptors for chemokines have recently been identified as essential co-receptors required for the process of HIV entry into target cells (75). Macrophages and their counterparts within the central nervous system, the microglial cells, also express cell surface CD4 and provide targets for HIV infection. As macrophages are more resistant to the cytopathic consequences of HIV infection than are CD4+ T cells and are widely distributed throughout the body, they may play critical roles in persistence of HIV infection by providing reservoirs of chronically infected cells. Although most of the immunologic and virologic assessments of HIV-infected persons have focused on studies of peripheral blood lymphocytes, these cells represent only approximately 2% of the total lymphocyte population in the body. The importance of the lymphoid organs, which contain the majority of CD4+ T cells, has been highlighted by the finding that the concentrations of virus and percentages of HIV-infected CD4+ T cells are substantially higher in lymph nodes (where immune responses are generated and where activated and proliferating CD4+ T cells that are highly susceptible to HIV infection are prevalent) than in peripheral blood (3,4,48). Thus, although the depletion of CD4+ T cells after HIV infection is most readily revealed by sampling peripheral blood, damage to the immune system is exacted in lymphoid organs throughout the body (3,4). For as yet unidentified reasons, gradual destruction of normal lymph node architecture occurs with time, which probably compromises the ability of an HIV-infected person to generate effective immune responses and replace CD4+ T cells already lost to HIV infection through the expansion of mature T cell populations in peripheral lymphoid tissues. The thymus is also an early target of HIV infection and damage, thereby limiting the continuation of effective T cell production even in younger persons in whom thymic production of CD4+ T cells is active (76,77). Thus, in both adults and children, HIV infection compromises both of the potential sources of T cell production, so the rate of T cell replenishment cannot continue indefinitely to match cell loss. Consequently, total CD4+ T cell numbers may decline inexorably in HIV-infected persons. After initial infection, the pace at which immunodeficiency develops and the attendant susceptibility to OIs which arise are associated with the rate of decline of CD4+ T cell counts (11,26,27). The rate at which CD4+ T cell counts decline differs considerably from person to person and is not constant throughout all stages of the infection. Acceleration in the rate of decline of CD4+ T cells heralds the progression of disease. The virologic and immunologic events that occur around this time are poorly understood, but increasing rates of HIV replication, the emergence of viruses demonstrating increased cytopathic effects for CD4+ T cells, and declining host cell-mediated anti-HIV immune responses are often seen (12,78). For as yet unknown reasons, host compensatory responses that preserve the homeostasis of total T cell levels (CD4+ plus CD8+ T cells) appear to break down in HIV-infected persons approximately 1-2 years before the development of AIDS, resulting in net loss of total T cells in the peripheral blood, and signaling immune system collapse (79). Although the progression of HIV disease is most readily gauged by declining CD4+ T cell numbers, evidence indicates that the sequential loss of specific types of immune responses also occurs (80-82). Memory CD4+ T cells are known to be preferential targets for HIV infection, and early loss of CD4+ memory T cell responses is observed in HIV-infected persons, even before there are substantial decreases in total CD4+ T cell numbers (80,81). With time, gradual attrition of antigen-specific CD4+ T cell-dependent immune recognition may limit the repertoire of immune responses that can be mounted effectively and so predispose the host to infection with opportunistic pathogens (82). HIV Replication Rates in Infected Persons Can Be Accurately Gauged By Measurement of Plasma HIV Concentrations Until recently, methods for monitoring HIV replication (commonly referred to as viral load) in infected persons were either hampered by poor sensitivity and reproducibility or were so technically laborious that they could not be adapted for routine clinical practice. However, new techniques for sensitive detection and accurate quantification of HIV RNA levels in the plasma of infected persons provide extremely useful measures of active virus replication (1,2,19,20,37,41-43). HIV RNA in plasma is contained within circulating virus particles or virions, with each virion containing two copies of HIV genomic RNA. Plasma HIV RNA concentrations can be quantified by either target amplification methods (e.g., quantitative RT polymerase chain reaction {RT-PCR}, Amplicor HIV Monitor (TM) assay, Roche Molecular Systems; or nucleic acid sequence-based amplification, {NASBA (R)}, NucliSens (TM) HIV-1 QT assay, Organon Teknika) or signal amplification methods (e.g., branched DNA {bDNA}, Quantiplex (TM) HIV RNA bDNA assay, Chiron Diagnostics) (42,43). The bDNA signal amplification method (41) amplifies the signal obtained from a captured HIV RNA target by using sequential oligonucleotide hybridization steps, whereas the RT-PCR and NASBA (R) assays use enzymatic methods to amplify the target HIV RNA into measurable amounts of nucleic acid product (41-43). Target HIV RNA sequences are quantitated by comparison with internal or external reference standards, depending upon the assay used. Versions of both types of assays are now commercially available, and the Amplicor assay was recently approved by the Food and Drug Administration for assessment for risk of disease progression and monitoring of antiretroviral therapy in HIV-infected persons. Target amplification assays are more sensitive (400 copies HIV RNA/mL plasma) than the first generation bDNA assay (10,000 copies HIV plasma), but the sensitivity of the bDNA assay has recently been improved (500 copies HIV RNA/mL plasma). More sensitive versions of each of these assays are currently in development (detection limits 20-100 copies/mL) and will likely be commercially available in the future. All of the commercially available assays can accurately quantitate plasma HIV RNA levels across a wide range of concentrations (so-called dynamic range). Although the results of the three assays (i.e., the RT-PCR, NASBA (R), and bDNA) are strongly correlated, the absolute values of HIV RNA measured in the same plasma sample using two different assays can differ by twofold or more (44-46). Until a common standard is available that can be used to normalize values obtained with different assay methods, it is advisable to choose one assay method consistently when HIV RNA levels in infected persons are monitored for use as a guide in making therapeutic decisions. The performance characteristics and recommended collection methods for the individual HIV RNA assays are provided (Table_1). For reliable results, it is essential that the recommended procedures be followed for collection and processing of blood to prepare plasma for HIV RNA measurements. Different plasma HIV RNA assays require different plasma volumes (an important consideration in infants and in young children). These assays are best performed on plasma specimens prepared from blood obtained in collection tubes containing specific anticoagulants (e.g., ethylenediaminetetraacetic acid {EDTA} or acid-citrate-dextran {ACD}) (Table_1) (44-46). Quantitative measurement of plasma HIV RNA levels can be expressed in two ways: a) the number of copies/mL of HIV RNA and b) the logarithm (to the base 10) of the number of copies/mL of HIV RNA. In clinically stable, HIV-infected adults, results obtained by using commercially available plasma HIV RNA assays can vary by approximately threefold (0.5 log10) in either direction on repeated measurements obtained within the same day or on different days (35,36). Factors influencing the variation seen in plasma HIV RNA assays include biological fluctuations and those introduced by the performance characteristics of the particular assay (35,36,44-46). Variability of current plasma HIV RNA assays is greater toward their lower limits of detection and consequently changes greater than 0.5 log10 HIV RNA copies can be seen near the assay detection limits without changes in clinical status (35). Differences greater than 0.5 log10 copies on repeated measures of plasma HIV RNA likely reflect biologically and clinically relevant changes. Increased variance toward the limit of assay detection presents an important consideration as the recommended target of suppression of HIV replication by antiretroviral therapy is now defined as being HIV RNA levels below the detection limit of plasma HIV RNA assays. Immune system activation (by immunizations or intercurrent infections) can lead to increased numbers of activated CD4+ T cells, and thereby result in increased levels of HIV replication (reflected by significant elevations of plasma HIV RNA levels from their baseline values) that may persist for as long as the inciting stimulus remains (32-34). Therefore, measurements obtained surrounding these events may not reflect a patient's actual steady-state level of plasma HIV RNA. Unlike CD4+ T cell count determinations, plasma HIV RNA levels do not exhibit diurnal variation (26,36). Within the large dynamic range of plasma HIV RNA levels that can be measured (varying over several log10 copies), the observed level of assay variance is low (Table_1). Measurement of two samples at baseline in clinically stable patients has been recommended as a way of reducing the impact of the variability of plasma HIV RNA assays (19), and recent data support this approach (22). The level of viremia, as measured by the amount of HIV RNA in the plasma, accurately reflects the extent of virus replication in an infected person (1,2,20,37). Although the lymphoid tissues (e.g., lymph nodes and other compartments of the reticuloendothelial system) provide the major sites of active virus production in HIV-infected persons, virus produced in these tissues is released into the peripheral circulation where it can be readily sampled (3,4,48). Thus, plasma HIV RNA concentrations reflect the level of active virus replication throughout the body, although it is not known whether specific compartments (e.g., the central nervous system {CNS}) represent sites of infection that are not in direct communication with the peripheral pool of virus. The Magnitude of HIV Replication in Infected Persons Determines Their Rate of Disease Progression Plasma HIV RNA can be detected in virtually all HIV-infected persons although its concentration can vary widely depending on the stage of the infection (Figure_1) and on incompletely understood aspects of the host-virus interactions. During primary infection in adults when there are numerous target cells susceptible to HIV infection without a countervailing host immune response, concentrations of plasma HIV RNA can exceed 107 copies/mL (83). HIV disseminates widely throughout the body during this period, and many newly infected persons display symptoms of an acute viral illness, including fever, fatigue, pharyngitis, rash, myalgias, and headache (84-86). Coincident with the emergence of antiviral immune responses, concentrations of plasma HIV RNA decline precipitously (by 2 to 3 log10 copies or more). After a period of fluctuation, often lasting 6 months or more, plasma HIV RNA levels usually stabilize around a so-called set-point (5,6,10,27,31,86). The determinants of this set-point are incompletely understood but probably include the number of susceptible CD4+ T cells and macrophages available for infection, the degree of immune activation, and the tropism and replicative vigor (fitness) of the prevailing HIV strain at various times following the initial infection, as well as the effectiveness of the host anti-HIV immune response. In contrast to adults, HIV-infected infants often have very high levels of plasma HIV RNA that decline slowly with time and do not reach set-point levels until more than a year after infection (14-18). Different infected persons display different steady-state levels of HIV replication. When populations of HIV-infected adults are studied in a cross-sectional manner, an inverse correlation between plasma HIV RNA levels and CD4+ T cell counts is seen (87,88). However, at any given CD4+ T cell count, plasma HIV RNA concentrations show wide interindividual variation (87,88). In established HIV infection, persistent concentrations of plasma HIV RNA range from less than 200 copies/mL in extraordinary persons who have apparently nonprogressive HIV infection to greater than 106 copies/mL in persons who are in the advanced stages of immunodeficiency or are at risk for very rapid disease progression. In most HIV-infected and untreated adults, set-point plasma HIV RNA levels range between 103 and 105 copies/mL. Persons who have higher steady-state set-point levels of plasma HIV RNA generally lose CD4+ T cells more quickly, progress to AIDS more rapidly, and die sooner than those with lower HIV RNA set-point levels (5-7,10,27) (Figure_2, Figure_3, Figure_4). Once established, set-point HIV RNA levels can remain fairly constant for months to years. However, studies of populations of HIV-infected persons suggest a gradual trend toward increasing HIV RNA concentrations with time after infection (10). Within individual HIV-infected persons, rates of increase of plasma HIV RNA levels can change gradually, abruptly, or hardly at all (10). Progressively increasing plasma HIV RNA concentrations can signal the development of advancing immunodeficiency, regardless of the initial set-point value (10,75). Plasma HIV RNA levels provide more powerful predictors of risk of progression to AIDS and death than do CD4+ T cell levels; however, the combined measurement of the two values provides an even more accurate method to assess the prognosis of HIV-infected persons (27). The relationship between baseline HIV RNA levels measured in a large cohort of HIV-infected adults and their subsequent rate of CD4+ T cell decline is shown (Figure_3) (27). Progressive loss of CD4+ T cells is observed in all strata of baseline plasma HIV RNA concentrations, but substantially more rapid rates of decline are seen in persons who have higher baseline levels of plasma HIV RNA (Figure_3) (27). Likewise, a clear gradient in risk for disease progression and death is seen with increasing baseline plasma HIV RNA levels (5,6,10,27) (Figure_2 and Figure_4). HIV Replicates Actively at All Stages of the Infection The steady-state level of HIV RNA in the plasma is a function of the rates of production and clearance (i.e., the turnover) of the virus in circulation (1,2,20,21,37). Effective antiretroviral therapy perturbs this steady state and allows an assessment of the kinetic events that underlie it. Thus, virus clearance, the magnitude of virus production, and the longevity of virus-producing cells can all be measured. Recent studies in which measurements of virus and infected-cell turnover were analyzed in this way in persons who had moderate to advanced HIV disease have demonstrated that a very dynamic process of virus production and clearance underlies the seemingly static steady-state level of HIV virions in the plasma (1,2,20,21,37). Within 2 weeks of initiation of potent antiretroviral therapy, plasma HIV RNA levels usually fall to approximately 1% of their initial values (20,37) (Figure_5). The slope of this initial decline reflects the clearance of virus from the circulation and the longevity of recently infected CD4+ T cells and is remarkably constant among different persons (1,2,20,37). The half-life of virions in circulation is exceedingly short -- less than 6 hours. Thus, on average, half of the population of plasma virions turns over every 6 hours or less. Given such a rapid rate of virus clearance, it is estimated that 109 to 1010 (or more) virions must be produced each day to maintain the steady-state plasma HIV RNA levels typically found in persons who have moderate to advanced HIV disease (20). When new rounds of virus replication are blocked by potent antiretroviral drugs, virus production from the majority of infected cells (approximately 99%) continues for only a short period, averaging approximately 2 days (1,2,20,37). HIV-infected CD4+ T cells are lost, presumably as the result of direct cytopathic effects of virus infection, with an average half-life of an infected cell being approximately 1.25 days (20). The estimated generation time of HIV (the time from release of a virion until it infects another cell and results in the release of a new generation of virions) is approximately 2.5 days, which implies that the virus is replicating at a rate of approximately 140 or more cycles per year in an infected person (20,21). Thus, at the median period between initial infection and the diagnosis of AIDS, each virus genome present in an HIV-infected person is removed by more than a thousand generations from the virus that initiated the infection. After the initial rapid decline in plasma HIV RNA levels following initiation of potent antiretroviral therapy, a slower decay of the remaining 1% of initial plasma HIV RNA levels is observed (37) (Figure_5). The length of this second phase of virus decay differs among different persons, lasting approximately 8-28 days. Most of the residual viremia is thought to arise from infected macrophages that are lost over an average half-life of about 2 weeks, whereas the remainder is produced following activation of latently infected CD4+ T cells that decay with an average half-life of about 8 days. Within 8 weeks of initiation of potent antiretroviral therapy (in previously untreated patients), plasma HIV RNA levels commonly fall below the level of detection of even the most sensitive plasma HIV RNA assays available (sensitivity of 25 copies HIV RNA/mL), indicating that new rounds of HIV infection are profoundly suppressed (Figure_5) (37). Fortunately, this level of suppression of HIV replication appears to have been maintained for more than 16 months in most patients who adhere to effective combination antiretroviral drug regimens (39). However, even this marked pharmacologic interference of HIV replication has not yet been reported to eradicate an established infection. Those rare persons who have been studied after having stopped effective combination antiretroviral therapy following months with undetectable levels of plasma HIV RNA have all shown rapid rebounds in HIV replication. Furthermore, infectious HIV can still be isolated from CD4+ T cells obtained from antiretroviral treated persons whose plasma HIV RNA levels have been suppressed to undetectable levels (less than 50 copies/mL) for 2 years or more (49,50). Viruses recovered from these persons were demonstrated to be sensitive to the antiretroviral drugs used, indicating that a reservoir of latently infected resting CD4+ T cells exists that can maintain HIV infection for prolonged periods even when new cycles of virus replication are blocked. It is not known whether additional reservoirs of residual HIV infection exist in infected persons that can permit persistence of HIV infection despite profound inhibition of virus replication by effective combination antiretroviral therapies (37,47,48). HIV infection within the CNS represents an additional potential sanctuary for virus persistence, as many of the antiretroviral drugs now available do not efficiently cross the blood-brain barrier. Active HIV Replication Continuously Generates Viral Variants That are Resistant to Antiretroviral Drugs HIV replication depends on a virally encoded enzyme, RT (an RNA-dependent DNA polymerase) that copies the single-stranded viral RNA genome into a double-stranded DNA in an essential step in the virus life cycle (21). Unlike cellular DNA polymerases used to copy host cell chromosomal DNA during the course of cell replication, RT lacks a 3' exonuclease activity that serves a "proofreading" function to repair errors made during transcription of the HIV genome. As a result, the HIV RT is an "error-prone" enzyme, making frequent errors while copying the RNA into DNA and giving rise to numerous mutations in the progeny virus genomes produced from infected cells. Estimates of the mutation rate of HIV RT predict that an average of one mutation is introduced in every one to three HIV genomes copied (21,89). Additional variation is introduced into the replicating population of HIV variants as a result of genetic recombination that occurs during the process of reverse transcription via template-switching between the two HIV RNA molecules that are included in each virus particle (21,90). Many mutations introduced into the HIV genome during the process of reverse transcription will compromise or abolish the infectivity of the virus; however, other mutations are compatible with virus infectivity. In HIV-infected persons, the actual frequency with which different genetic variants of HIV are seen is a function of their replicative vigor (fitness) and the nature of the selective pressures that may be acting on the existing swarm of genetic variants present (21). Important selective pressures that may exist in HIV-infected persons include their anti-HIV immune responses, the availability of host cells that are susceptible to virus infection in different tissues, and the use of antiretroviral drug treatments. The rate of appearance of genetic variants of HIV within infected persons is a function of the number of cycles of virus replication that occurs during a person's infection (20,21). That numerous rounds of HIV replication are occurring daily in infected persons provides the opportunity to generate large numbers of variant viruses, including those that display diminished sensitivity to antiretroviral drugs. A mutation is probably introduced into every position of the HIV genome many times each day within an infected person, and the resulting HIV variants may accumulate within the resident virus population with successive cycles of virus replication (21). As a result of the great genetic diversity of the resident population of HIV, viruses harboring mutations that confer resistance to a given antiretroviral drug, and perhaps multiple antiretroviral drugs, are likely to be present in HIV-infected persons before antiretroviral therapy is initiated (21). Indeed, mutations that confer resistance to nucleoside analog RT inhibitors, NNRTIs, and PIs have been identified in HIV-infected persons who have never been treated with antiretroviral drugs (61,91,92). Once drug therapy is initiated, the pre-existing population of drug-resistant viruses can rapidly predominate. For drugs such as 3TC and nevirapine (and other NNRTIs), a single nucleotide change in the HIV RT gene can confer 100- to 1,000-fold reductions in drug susceptibility (1,61,93-95). Although these agents may be potent inhibitors of HIV replication, the antiretroviral activity of these drugs when used alone is largely reversed within 4 weeks of initiation of therapy due to the rapid outgrowth of drug-resistant variants (1,61,93-95). The rapidity with which drug-resistant variants emerge in this setting is consistent with the existence of drug-resistant subpopulations of HIV within infected patients before to the initiation of treatment (21,61). Because treatment with many of the available antiretroviral drugs selects for HIV variants that harbor the same or related mutations, specific treatments can select for the outgrowth of HIV variants that are resistant to drugs with which the patient has not been treated (referred to as cross-resistance) (96,97). Drug-resistant viruses that emerge during drug therapy are predicted to replicate less well (are less fit) than their wild-type counterparts and are expected to attain lower steady-state levels of viral load than are present before the initiation of therapy (21). Evidence for such decreased fitness of drug-resistant viruses has been gleaned from studies of protease-inhibitor-treated or 3TC-treated patients, but this effect has not been apparent in NNRTI-treated patients (e.g., nevirapine or delavirdine) (1,61). Depending on its relative fitness, the drug-resistant variant can persist at appreciable levels even after the antiretroviral therapy that selected for its outgrowth is withdrawn. HIV variants resistant to nevirapine can persist for more than a year after withdrawal of nevirapine treatment (61). Zidovudine-resistant HIV variants and variants resistant to both zidovudine and nevirapine have also been shown to persist in infected persons and to replicate well enough to be transmitted from one person to another (98). Because HIV variants that are resistant to PIs often appear to be less fit than drug-sensitive viruses, their prevalence in patients who develop PI resistance may decline after withdrawal of the drug. However, although such variants may decline after drug withdrawal, they also may persist in patients at higher levels than their original levels and can be rapidly selected for should the same antiretroviral agent (or a PI demonstrating cross-resistance) be used again (97). The definition of mutations associated with resistance to specific antiretroviral drugs and the advent of genetic methods to detect drug-resistant variants in treated patients have raised the possibility of screening HIV-infected patients for the presence of HIV variants as a tool to guide therapeutic decisions (92,99). However, this approach must be considered experimental and may prove very difficult to implement because of the complex patterns of mutations that increase resistance to some antiretroviral agents. Furthermore, the prevalence of clinically important populations of drug-resistant variants in many HIV-infected persons is likely to be below the level of detection of the available assays, thus potentially creating falsely optimistic predictions of drug efficacy (21,61). Combination Antiretroviral Therapy That Suppresses HIV Replication to Undetectable Levels Can Delay or Prevent the Emergence of Drug-Resistant Viral Variants Current strategies for antiretroviral therapy are much more effective than those previously available, and the efficacy of these approaches confirms predictions emerging from fundamental studies of the biology of HIV infection. Several important principles have emerged from these studies that can be used to guide the application of antiretroviral therapies in clinical practice:
Antiretroviral Therapy-Induced Inhibition of HIV Replication Predicts Clinical Benefit As active HIV replication is directly linked to the progressive depletion of CD4+ T cell populations, reduction in levels of virus replication by antiretroviral drug therapy is predicted to correlate with the clinical benefits observed in treated patients. Data from an increasing number of clinical trials of antiretroviral agents provide strong support for this prediction and indicate that greater clinical benefit is obtained from more profound suppression of HIV replication (9,13,23,38-40,56). For example, virologic analyses from ACTG 175 (a study of zidovudine or didanosine monotherapy compared with combination therapy with zidovudine plus either didanosine or zalcitabine) indicate that a reduction in plasma HIV RNA levels to 1.0 log below baseline at 56 weeks after initiation of therapy was associated with a 90% reduction in risk of progression of clinical disease (13). In a pooled analysis of seven different ACTG studies, durable suppression of plasma HIV RNA levels to less than 5,000 copies of HIV RNA/mL between 1 and 2 years after initiation of treatment was associated with an average increase in CD4+ T cell levels of approximately 90 cells/mm3 (24). Patients whose plasma HIV RNA levels failed to be stably suppressed to less than 5,000 copies/mL showed progressive decline in CD4+ T cell counts during the same period (24). Decreases in plasma HIV RNA levels induced by antiretroviral therapy provide better indicators of clinical benefit than CD4+ T cell responses (9,13,24). Furthermore, in patients who have advanced HIV disease, clinical benefit correlates with treatment-induced decreases in plasma HIV RNA levels, even when CD4+ T cell increases are not seen. The failure to observe CD4+ T cell increases in some treated patients despite suppression of HIV replication may reflect irreversible damage to the regenerative capacity of the immune system in the later stages of HIV disease. The most extensive data on the relationship between the magnitude of suppression of HIV replication induced by antiretroviral therapy and the degree of improved clinical outcome were generated during studies of nucleoside analog RT inhibitors used alone or in combination (9,13,24). These treatments yield less profound and less durable suppression of HIV replication than currently available combination therapy regimens that include potent PIs (and that are able to suppress HIV replication to levels below the detection limits of plasma HIV RNA assays) (23,37,39). Thus, it is likely that the relationship between suppression of HIV replication and clinical benefit will become even more apparent as experience with potent combination therapies accumulates. Repair of immune system function may be incomplete following effective inhibition of continuing HIV replication and damage by antiretroviral drug therapy. As discussed in the preceding principles, disease progression in HIV-infected patients results from active virus replication that inflicts chronic damage upon the function of the immune system and its structural elements, the lymphoid tissues. Because of the clonal nature of the antigen-specific immune response, in the absence of generation of immunocompetent CD4+ T cells from immature progenitor cells, it is likely that T cell responses may not be regained once lost, even if new rounds of HIV infection can be stopped by effective antiretroviral therapy (80,82,101). Similarly, it is not known if the damaged architecture of the lymphoid organs seen in persons with moderate to advanced HIV disease can be repaired following antiretroviral drug therapy. Should the residual proliferative potential of CD4+ and CD8+ T cells decline with increased duration of HIV infection and the magnitude of the cumulative loss and regeneration of lymphocyte populations, late introduction of antiretroviral therapy may have limited ability to reconstitute levels of functional lymphocytes. Thus, it is believed that the initiation of antiretroviral therapy before extensive immune system damage has occurred will be more effective in preserving and improving the ability of the HIV-infected person to mount protective immune responses. Few reliable methods are now available to assess the integrity of immune responses in humans. However, the application of specific methods to the study of immune responses in HIV-infected patients before and after initiation of antiretroviral therapy indicates that immunologic recovery is incomplete even when HIV replication falls to undetectable levels. CD4+ T cell levels do not return to the normal range in most antiretroviral drug-treated patients, and the extent of CD4+ T cell increase is typically more limited when therapy is started in the later stages of HIV disease (82). Recent evidence indicates that the repertoire of antigen-specific CD4+ T cells becomes progressively constricted with declining T cell numbers (82). In persons who have evidence of a restricted T cell repertoire, antiretroviral therapy can increase total CD4+ T cell numbers but fails to increase the diversity of antigen recognition ability (82). It is not yet known if expansion of a constricted CD4+ T cell repertoire of antigen recognition might be seen with longer-term follow-up of such persons. Reports of OIs occurring in antiretroviral-treated patients at substantially higher CD4+ T cell counts than those typically associated with susceptibility to the specific opportunistic infections raise the concern that restoration of protective immune responses may be incomplete, even when effective suppression of continuing HIV replication is achieved (102). However, other reports describe instances in which the clinical symptoms or signs of preexisting OIs were ameliorated (103-105), or in which new inflammatory responses to preexisting, but subclinical, OIs became manifest following initiation of effective combination antiretroviral therapy (106,107). These observations indicate that some improvement in immune function may be possible, even in patients who have advanced HIV disease, if sufficient numbers of pathogen-specific CD4+ T cells are still present when effective antiretroviral therapy is begun. The extent to which antiretroviral therapy can restore immune function when initiated in persons at varying stages of HIV disease is currently unknown but represents an essential question for future research. References
* Information included in these principles may not represent FDA approval or approved labeling for the particular products or indications in question. Specifically, the terms "safe" and "effective" may not be synonymous with the FDA-defined legal standards for product approval. Appendices Table_1 TABLE. Characteristics of plasma HIV RNA assays Figure_1 FIGURE 1. Generalized time course of HIV infection and disease Figure_2 FIGURE 2. AIDS-free survival by baseline plasma HIV RNA and CD4+ T cell levels Figure_3 FIGURE 3. Association between rates of decline of CD4+ T cell counts and baseline plasma HIV RNA level Figure_4 FIGURE 4. Probability of AIDS by baseline HIV RNA level and CD4+ T cell count Figure_5 FIGURE 5. Rate of decline of plasma HIV RNA concentration after initiation of potent combination antiretroviral therapy Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE. Characteristics of plasma HIV RNA assays *
==================================================================================================
Observed intra-assay
Linear dynamic (copies/ mL) standard Preferred
Assay range + (copies/ mL) deviation range (log10) & anticoagulant
-----------------------------------------------------------------------------------
2 5.2
RT-PCR @ 4 x 10 -10 <0.15- 0.33 ACD/EDTA **
2 6
bDNA ++ 5 x 10 -1. 6 x 10 0.08- 0.2 EDTA **
2 7
NASBA r && 4 x 10 -4 x 10 0.13- 0.23 ACD/EDTA/HEP **
-----------------------------------------------------------------------------------
* More sensitive versions of each of these assays (detection limits 20-100 HIV RNA copies/ mL)
are currently in development and will likely be commercially available in the future.
+ Higher values can be measured with dilution of the specimen into the linear dynamic range
for each assay.
& Ranges are representative of those obtained in comparative analyses of plasma HIV RNA
assays (44-46). Plasma HIV RNA assays tend to be more variable at or near the limit of
quantitation. Thus, the significance of changes in HIV RNA levels at the lowest levels of
quantitation for a given assay should be evaluated in light of this increased variability.
@ Amplicor HIV Monitor (TM) assay (Roche Molecular Systems, Alameda, CA).
** ACD = acid citrate dextran (citrate; yellow-top tube); EDTA = ethylenediaminetetraacetic acid
(purple-top tube); HEP = heparin (green-top tube).
++ Quantiplex (TM) HIV RNA bDNA assay (Chiron Diagnostics, Emeryville, CA).
&& NucliSens (TM) HIV-1 QT assay (Organon Teknika, Boxtel, The Netherlands).
==================================================================================================
Return to top. Figure_1 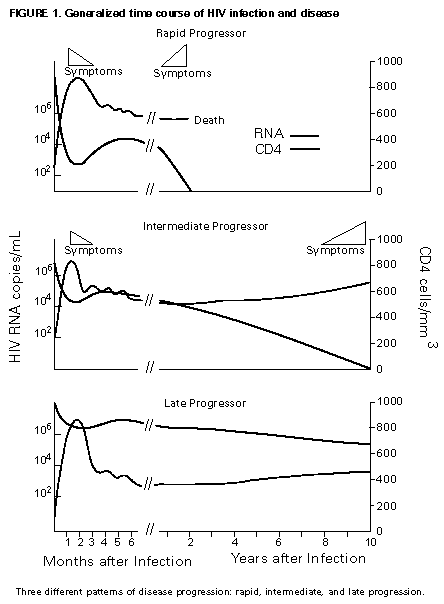 Return to top. Figure_2 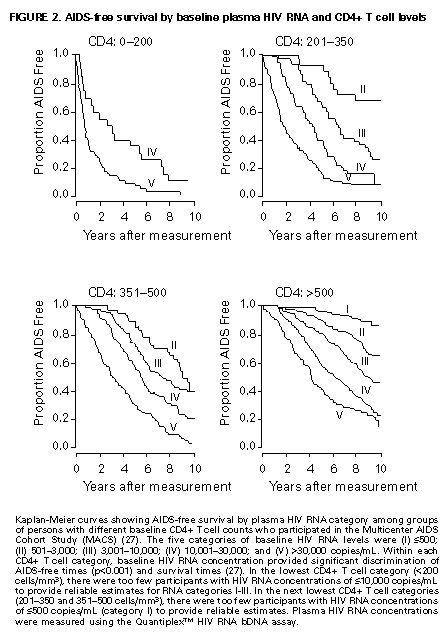 Return to top. Figure_3 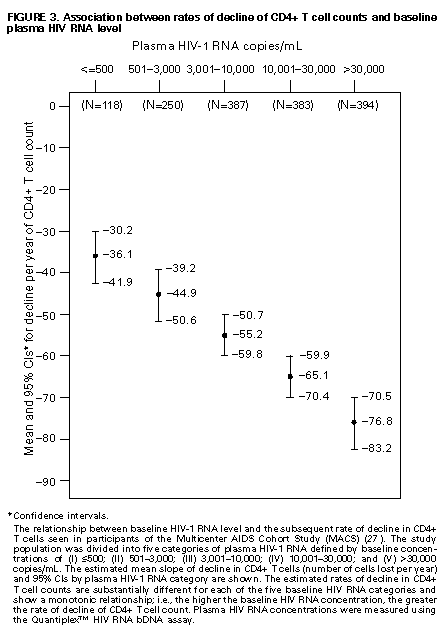 Return to top. Figure_4 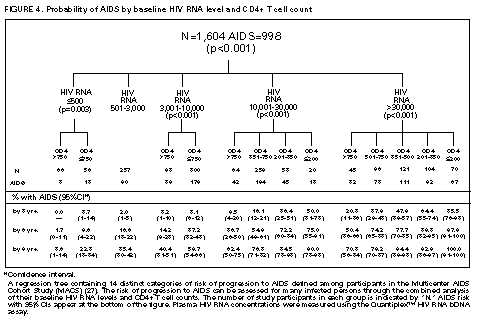 Return to top. Figure_5 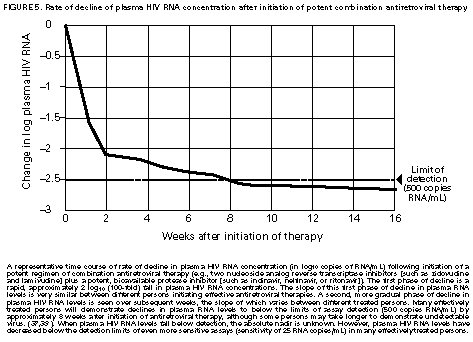 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/05/98 |
|||||||||
This page last reviewed 5/2/01
|