 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Progress Toward Poliomyelitis Eradication -- South East Asia Region, 1995-1997In 1988, the World Health Assembly established the goal of global poliomyelitis eradication by the year 2000. Since then, substantial progress has been reported from all World Health Organization (WHO) regions by implementing strategies to prevent, detect, and interrupt transmission of poliovirus (1). In WHO's South-East Asia Region * (SEAR), the successful application of these strategies has resulted in a 96% decrease in the number of annually reported polio cases during 1988-1996 (from 25,711 cases to 1116 cases). Acceleration of intensified surveillance continues to be critically important for identifying the remaining reservoirs of poliovirus circulation for targeted mass vaccination campaigns. This report summarizes data on progress in SEAR toward polio eradication as of April 1, 1997, and updates previous reports (2-4). Vaccination Coverage Routine vaccination. During 1986-1995, all countries in SEAR implemented the Expanded Program on Immunization. During 1986-1990, overall coverage in SEAR with three doses of oral poliovirus vaccine (OPV3) among children aged less than 1 year increased from 42% to 82% and, during 1991-1995, coverage ranged from 85% to 91%. Supplementary vaccination. In 1994, annual National Immunization Days (NIDs) ** were first held in Thailand, followed in 1995 by Bangladesh, Bhutan, India, Indonesia, and Sri Lanka, and in 1996 by the Democratic People's Republic (DPR) of Korea, Myanmar, and Nepal. South Asia Association for Regional Cooperation (SAARC) member countries in the WHO South-East Asia and Eastern Mediterranean regions coordinated NIDs during December 1996-January 1997. In SEAR, supplementary doses of OPV were administered during this period to 165 million children aged less than 5 years during NIDs conducted simultaneously in six (Bangladesh, Bhutan, India, Myanmar, Nepal, and Thailand) of the region's 10 countries. Incidence of Polio During 1988-1996, the annual number of reported polio cases in SEAR decreased by 96% (from 25,711 cases to 1116 cases). The cases reported in SEAR in 1996 (1116) accounted for 30% of the worldwide burden of paralytic poliomyelitis (3755) and, in 1988 and 1994, for 73% and 67% of cases worldwide, respectively. Five countries in the region (Bangladesh, India, Indonesia, Myanmar, and Nepal) accounted for 99% (1109 of 1116) of the total number of cases reported in the region in 1996 (Table_1). From 1994 (implementation of the first NIDs in the region {Thailand}) to 1996, reported polio cases decreased by 78% (from 5118 cases to 1116 cases). The substantial decline in reported cases primarily reflects improved control of polio in India (1996 population: 952,969,000 {76% of the region's population}). Following the implementation of India's first NIDs during December 1995-January 1996, reported cases decreased by 69% from 1995 to 1996 (from 3263 cases to 1005 cases) (Figure_1). During 1996, six other countries reported polio cases, including Indonesia (63 cases {6% of the regional total}), Bangladesh (24 cases {2%}), Myanmar (11 {1%}), Nepal (nine {less than 1%}), DPR Korea (six {less than 1%}), and Thailand (one {less than 1%}); three countries (Bhutan, Maldives, and Sri Lanka) reported zero polio cases. Bhutan (1996 population: 1,634,000; last reported polio case was in 1986) and Maldives (1996 population: 251,000; last reported case {imported} was in 1994) have implemented "zero case" reporting *** for cases of acute flaccid paralysis (AFP) from all reporting units. Sri Lanka, which has maintained intensified surveillance **** since 1991, last reported polio cases in 1993. Surveillance By 1995, all SEAR countries were conducting surveillance for clinically confirmed paralytic poliomyelitis; however, only two countries (Sri Lanka and Thailand) had established surveillance for AFP. By 1996, all member countries had initiated procedures for the mandatory reporting and investigation of all cases of AFP in children aged less than 15 years. In some countries (Bangladesh, India, Indonesia, Myanmar, and Nepal), intensive training has been instituted for public health officials and physicians in clinical practice regarding immediate reporting and investigation of all AFP cases. In 1996, Sri Lanka was the only country to achieve or exceed the WHO-established minimum AFP reporting rate indicative of a sensitive surveillance system (greater than or equal to 1 non-polio AFP case per 100,000 population aged less than 15 years); the nonpolio AFP rate reported for Sri Lanka was 1.7. No country in the region has achieved the WHO-recommended target of two stool specimens collected at a 24- to 48-hour interval within 14 days of paralysis onset from at least 80% of AFP cases; the proportion of cases in 1996 with two stools collected within 14 days ranged from 16% (Bangladesh) to 61% (Sri Lanka). Virologic Investigations Enterovirus isolation, identification, and intratypic differentiation is performed by the SEAR Poliovirus Laboratory network ***** on stool specimens collected from AFP cases. Based on an expected nonpolio AFP rate of at least 1 case per 100,000 population aged less than 15 years, the minimum expected number of cases in SEAR would be 5033 per year. However, in 1996, a total of 1381 AFP cases were reported from all countries in the region; of these, 1116 were classified as confirmed polio. Virologic investigations were conducted for 978 (71%) of the 1381 reported AFP cases. Of these, 106 (11%) were positive for wild poliovirus type 1; five (0.5%), for wild poliovirus type 2; and 11 (1%), for wild poliovirus type 3. In 1996, wild poliovirus was isolated from stool specimens from 126 AFP cases ****** in the region; of these, 110 (87%) were from India. Of these 110 isolates, 94 (85%) were wild poliovirus type 1; five (5%), wild poliovirus type 2; and 11 (10%), wild poliovirus type 3. India was the only country in the region from which wild poliovirus type 2 was isolated (from the northern states of New Delhi {one case}, Haryana {one}, and Uttar Pradesh {one}, and from the southern state of Tamil Nadu {two}). Wild poliovirus type 1 was isolated from Bangladesh (10 cases), Nepal (one), and Thailand (one). Reported by: South-East Asia Regional Office, New Delhi, India; Global Program for Vaccines and Immunization, World Health Organization, Geneva, Switzerland. Respiratory and Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Polio Eradication Activity, National Immunization Program, CDC. Editorial NoteEditorial Note: As of April 1, 1997, all SEAR countries with endemic polio had conducted from one to three successful NIDs, and coverage with OPV3 was greater than 95% in the target population in every country. The synchronized vaccination activities in the region resulted in the substantial decrease in reported polio cases in 1996 and the decrease in the proportion of total global polio cases accounted for by countries in SEAR. Because all 10 member countries plan to conduct NIDs during 1997-1998, such progress should be sustained in the region. During 1996-1997, coordination of NIDs in SEAR was enhanced by the active participation of SAARC, one of the partner organizations in the regional effort to eradicate polio. In addition, simultaneous coordination of NIDs or sub-NIDs by Pakistan (27 million children) in the Eastern Mediterranean Region and China (65 million children) in the Western Pacific Region resulted in vaccination of 257 million children in these contiguous countries with endemic polio. The political, financial, social, and logistic coordination needed to synchronize multi-national NIDs now should also be targeted toward strengthening surveillance both to prioritize eradication strategies and to document the eventual eradication of wild poliovirus. Although all countries in SEAR have implemented mandatory reporting of AFP cases, only one country in the region has achieved the recommended minimum rate of reporting. One priority is the development of highly sensitive epidemiologic and laboratory surveillance that meets standard performance criteria for identifying all remaining reservoirs of wild poliovirus. The persistent circulation of wild poliovirus in India during 1996 underscores the importance of establishing surveillance to enable precise identification of the virus reservoirs that can be targeted for routine or supplementary vaccination activities. Recent receipt of funds designated for surveillance from partner organizations, ******* including DANIDA (Danish government), Rotary International, U.S. Agency for International Development, and NORAD (Norwegian government), ensures that adequate financial resources are available to begin purchasing laboratory and field operations equipment, hire surveillance personnel, and support case investigation. Because some countries initiated polio-eradication strategies earlier than others, neighboring countries may reach the goal of elimination of wild poliovirus circulation at different times. Wild polioviruses circulated in countries or regions bordering emerging polio-free zones during 1995 and 1996, when cases of paralytic poliomyelitis occurred in children who resided in Myanmar but presented for treatment at a hospital in the neighboring province of Yunnan, China (5,6). To expedite rapid investigation of all such cases, all countries must ensure immediate notification of cases of AFP to the designated national authorities, neighboring countries, and relevant international organizations. To achieve the goal of global eradication of polio by 2000, international coordination of NIDs must be complemented by or integrated with cross-border coordination of surveillance. References
* Member countries are Bangladesh, Bhutan, Democratic People's Republic (DPR) of Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, and Thailand. ** Mass campaigns over a short period (days to weeks) in which two doses of OPV are administered to all children in the target age group, regardless of previous vaccination history, with an interval of 4-6 weeks between doses. *** Reporting the absence of cases. **** AFP rate of 1 case per 100,000 population aged less than 15 years; two stool specimens collected at an interval of 24-48 hours within 14 days of paralysis onset from greater than or equal to 80% of AFP cases; stool specimens tested in WHO-accredited laboratory. ***** Fifteen laboratories including three reference laboratories (in New Delhi, India; Colombo, Sri Lanka; and Nonthaburi, Thailand) and 12 national laboratories (in Dhaka, Bangladesh; Ahmedabad, Bangalore, Calcutta, Coonoor, Kasauli, Madras, and Mumbai, India; Bandung, Jakarta, and Surabaja, Indonesia; and Yangon, Myanmar). ****** Four cases of confirmed polio in DPR Korea had isolation of wild poliovirus in laboratories outside the SEAR laboratory network. ******* The polio-eradication initiative is supported by a coalition of organizations that include WHO, the United Nations Children's Fund (UNICEF), and other bilateral and multilateral organizations. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Number of reported cases of acute flaccid paralysis (AFP) and confirmed poliomyelitis* and key surveillance indicators, by country -- South-East Asia Region+, World Health Organization (WHO), 1995-1996
========================================================================================================================================================================================================================================================
1995 1996
--------------------------------------------------------------------------------------------------------------------- ---------------------------------------------------------------------------------------
No. confirmed cases AFP rate & % AFP cases with No. confirmed cases AFP rate % AFP cases with
-------------------------------- ------------------ two stool ------------------------------- -------------------- two stool
Country No. AFP cases Clinical Wild virus isolated Total Nonpolio specimens @ No. AFP cases Clinical Wild virus isolated Total Nonpolio specimens
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Bangladesh 108 49 2 0.23 0.12 NR ** 87 24 10 0.18 0.13 16
DPR Korea ++ 12 7 2 0.17 0.07 NR 13 6 4 0.19 0.10 43
India && 3263 3263 313 2.20 0 NR 1005 1005 110 0.30 0 NR
Indonesia 22 12 2 0.03 0.01 NR 68 63 0 0.11 0.01 42
Myanmar 7 7 0 0.04 0 NR 15 8 0 0.09 0.04 23
Nepal 15 9 7 0.16 0.06 NR 11 9 1 0.12 0.02 50
Sri Lanka 94 0 0 1.67 1.67 44 96 0 0 1.72 1.72 61
Thailand 122 2 2 0.74 0.73 75 86 1 1 0.56 0.52 40
Total 3643 3349 328 -- -- -- 1381 1116 126 -- -- --
--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
* A confirmed case of polio is defined as AFP and at least one of the following: 1) laboratory-confirmed wild poliovirus infection, 2) residual paralysis at 60 days, 3) death, or 4) no follow-up investigation at 60 days.
+ Bhutan and Maldives were excluded from this analysis because both are polio-free countries and have population sizes too small for meaningful analysis of nonpolio AFP data.
& Number of AFP cases per 100,000 population aged <15 years. Expected rate is>=1 case per 100,000 nonpolio AFP cases per year.
@ Two stool specimens collected at an interval of 24-48 hours within 14 days of paralysis onset from >=80% of AFP cases.
** Not reported.
++ Democratic People's Republic of Korea.
&& Source: Routine clinical polio surveillance, Ministry of Health and Family Welfare, Government of India. Implementation of AFP reporting is under way.
========================================================================================================================================================================================================================================================
Return to top. Figure_1 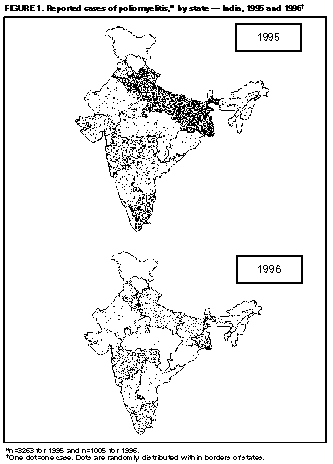 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|