 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Surveillance for Waterborne-Disease Outbreaks -- United States, 1993-1994Michael H. Kramer, M.D., M.P.H. (1, 2) Barbara L. Herwaldt, M.D., M.P.H. (2) Gunther F. Craun, P.E., D.E.E., M.P.H. (3) Rebecca L. Calderon, Ph.D., M.P.H. (4) Dennis D. Juranek, D.V.M., M.Sc. (2) (1) Epidemic Intelligence Service, Epidemiology Program Office, CDC (2) Division of Parasitic Diseases, National Center for Infectious Diseases, CDC (3) Global Consulting for Environmental Health, Radford, VA (4) Human Studies Division, National Health and Environmental Effects Laboratory, U.S. Environmental Protection Agency Summary Problem/Condition: Since 1971, CDC and the U.S. Environmental Protection Agency have maintained a collaborative surveillance system for collecting and periodically reporting data that relate to occurrences and causes of waterborne-disease outbreaks (WBDOs). Reporting Period Covered: This summary includes data for January 1993 through December 1994 and for previously unreported outbreaks in 1992. Description of the System: The surveillance system includes data about outbreaks associated with water intended for drinking (i.e., drinking water) and those associated with recreational water. State, territorial, and local public health departments are primarily responsible for detecting and investigating WBDOs and voluntarily reporting them to CDC on a standard form. Results: For the 2-year period 1993 1994, 17 states and one territory reported a total of 30 outbreaks associated with drinking water. These outbreaks caused an estimated 405,366 persons to become ill, including 403,000 from an outbreak of cryptosporidiosis in Milwaukee, the largest WBDO ever documented in the United States, and 2,366 from the other 29 outbreaks. No etiologic agent was identified for five (16.7%) of the 30 outbreaks. The protozoan parasites Giardia lamblia and Cryptosporidium parvum caused 10 (40.0%) of the 25 outbreaks for which the etiologic agent was identified. Two outbreaks of cryptosporidiosis occurred in large metropolitan areas (i.e., Milwaukee and Las Vegas/Clark County) and were associated with deaths among immunocompromised persons. The waterborne nature of these two outbreaks was not recognized until at least 2 weeks after the onset of the Milwaukee outbreak and until after the end of the Las Vegas outbreak. Campylobacter jejuni was implicated for three outbreaks and the following pathogens for one outbreak each: Shigella sonnei, Shigella flexneri, non-O1 Vibrio cholerae (in a U.S. territory; the vehicle was commercially bottled water), and Salmonella serotype Typhimurium (the outbreak was associated with seven deaths). Eight outbreaks of chemical poisoning were reported: three were caused by lead (one case each), two by fluoride, two by nitrate, and one by copper. Twenty (66.7%) of the 30 outbreaks were associated with a well-water source. Fourteen states reported a total of 26 outbreaks associated with recreational water, in which an estimated 1,714 persons became ill. Fourteen (53.8%) of these 26 were outbreaks of gastroenteritis. The etiologic agent in each of these 14 outbreaks was identified; 10 (71.4%) were caused by G. lamblia or C. parvum. Six of these 10 were associated with chlorinated, filtered pool water, and three with lake water. One of the latter was the first reported outbreak of cryptosporidiosis associated with the recreational use of lake water. Four outbreaks of lake water- associated bacterial gastroenteritis were reported, two caused by S. sonnei, one by S. flexneri, and one by Escherichia coli O157:H7. Nine outbreaks of hot tub-, whirlpool-, or swimming pool-associated pseudomonas dermatitis were reported. Two outbreaks of swimming pool-associated dermatitis had a suspected chemical etiology. The child who had the one reported case of primary amebic meningoencephalitis, caused by infection with Naegleria fowleri, died. Interpretation: The number of WBDOs reported annually has been similar for each year during 1987 1994, except for an increase in 1992. Protozoan parasites, especially C. parvum and G. lamblia, remain important etiologic agents of WBDOs. The outbreaks of cryptosporidiosis in Milwaukee and Las Vegas demonstrate that WBDOs can occur in large metropolitan areas. Surveillance methods are needed that expedite the detection of WBDOs and the institution of preventive measures (e.g., boil-water advisories). Actions Taken: Surveillance data that identify the types of water systems, their deficiencies, and the etiologic agents associated with outbreaks are used to evaluate the adequacy of current technologies for providing safe drinking and recreational water. In addition, they are used to establish research priorities and assist in improving water-quality regulations. INTRODUCTION National statistics on outbreaks associated with water intended for drinking (i.e., drinking water) have been available since 1920 (1). Since 1971, CDC, the U.S. Environmental Protection Agency (EPA), and the Council of State and Territorial Epidemiologists have maintained a collaborative surveillance system with the collection and periodic reporting of data on the occurrences and causes of waterborne-disease outbreaks (WBDOs) (2,3). The surveillance system includes data about outbreaks associated with drinking water and recreational water. This summary includes data for 1993 and 1994 and for previously unreported outbreaks in 1992. CDC's and EPA's efforts related to waterborne-disease surveillance have the following goals: a) to characterize the epidemiology of WBDOs; b) to identify the etiologic agents of WBDOs and to determine why the outbreaks occurred; c) to train public health personnel how to detect and investigate WBDOs; and d) to collaborate with local, state, federal, and international agencies on initiatives to prevent waterborne diseases. The data gathered through this surveillance system are useful for evaluating the adequacy of current technologies for providing safe drinking and recreational water. Surveillance information also influences research priorities and may lead to improved water-quality regulations. EPA REGULATIONS FOR DRINKING WATER Public water systems are regulated under the Safe Drinking Water Act of 1974 (4), as amended in 1986 (5). In 1989, to minimize microbial transmission, EPA promulgated the Surface Water Treatment Rule (SWTR; in effect since 1991 {6}) and in 1990 made the Total Coliform Rule more stringent (7,8). To limit the public's exposure to pathogens, the SWTR requires water utilities to disinfect surface water and groundwater that is at risk for contamination by surface water; human enteric viruses and Giardia lamblia cysts are target organisms for assessing the adequacy of treatment. In addition, utilities that use such source waters must provide filtration, unless specific criteria are met (e.g., with respect to turbidity, total or fecal coliforms in source water, and the operation of a watershed-control program to minimize contamination). The SWTR requires the monitoring of turbidity and various other parameters to demonstrate the adequacy of filtration and disinfection, respectively. The Total Coliform Rule currently requires, under certain conditions, periodic field inspections of water systems, testing water for Escherichia coli, and if water samples are positive for total coliforms, additional sampling to evaluate the water quality. No more than 5% of the samples collected in a given month can be positive for total coliforms. For systems in which <40 samples per month are required, no more than one sample can be positive. In addition, a maximum-contaminant-level goal for fecal coliforms or E. coli is specified. Repeated sampling and testing within 24 hours is required if these limits are exceeded. The Enhanced Surface Water Treatment Rule, proposed July 29, 1994 (9), and the Ground Water Disinfection Rule (to be proposed) also will address the prevention of waterborne diseases. The former may require additional treatment beyond that mandated by the SWTR for systems with source waters of poor quality. It also may include specific treatment requirements to ensure the removal of Cryptosporidium parvum oocysts. The latter rule will be designed to protect groundwater systems from microbial contamination, particularly by viruses. To emphasize the importance of having treatment plants operate optimally, EPA, in conjunction with operators of water-treatment plants, has initiated a voluntary program. Under this program, water utilities can have peer review, in addition to their self-assessment, of the performance of their treatment processes. Additionally, on February 10, 1994, EPA proposed the Information Collection Rule (10) for the collection of data about the presence of several pathogens and disinfection by-products (and selected precursors thereof) in water, as well as about the ability of treatment plants to remove these substances. This rule would require utilities that obtain water from surface-water sources and that provide service to greater than or equal to 100,000 persons to test, for 18 months, source water (in some instances, also finished water) for C. parvum and G. lamblia, total culturable viruses, total and fecal coliforms or E. coli, coliphages, and Clostridium perfringens. METHODS Sources of Data State, territorial, and local public health agencies have the primary responsibility for detecting and investigating WBDOs and voluntarily reporting them to CDC on a standard form (CDC form 52.12 {3}). CDC periodically requests reports from the state and territorial epidemiologists or from persons designated as coordinators of WBDO surveillance. When needed, additional information about water quality and treatment is obtained from the state's drinking-water agencies. Definitions of Terms * The surveillance system for WBDOs resembles that used for foodborne- disease outbreaks and differs from other systems in that the unit of analysis is an outbreak rather than an individual case of a particular disease. Two criteria must be met for an event to be defined as a WBDO. First, at least two persons must have experienced a similar illness after the ingestion of drinking water or after exposure to water used for recreational purposes. Second, epidemiologic evidence must implicate water as the probable source of the illness. The stipulation that at least two persons be ill is waived for single cases of laboratory-confirmed, primary amebic meningoencephalitis and for single cases of chemical poisoning if water-quality data indicate contamination by the chemical. If primary and secondary cases are distinguished on the outbreak-report form, only primary cases are included in the total number of cases. Outbreaks caused by contamination of water or ice at the point of use (e.g., a contaminated serving container) are not classified as WBDOs and are, instead, reported to the surveillance system for foodborne-disease outbreaks. Public water systems, classified as either community or noncommunity water systems, provide piped water to the public for general consumption and are regulated under the Safe Drinking Water Act. A community water system serves year-round residents of a community, subdivision, or mobile- home park that has greater than or equal to 15 service connections or an average of greater than or equal to 25 residents. A noncommunity water system serves an institution, industry, camp, park, hotel, or business that is used by the general public for greater than or equal to 60 days per year and has greater than or equal to 15 service connections or serves an average of greater than or equal to 25 persons. Of the approximately 215,000 water systems in the United States classified as public systems, 158,000 (73.5%) are noncommunity systems, serving transients (133,000 systems) and nontransients (25,000), and 57,000 (26.5%) are community systems (EPA, unpublished data, 1995). Individual water systems are defined as small water systems, not owned or operated by a water utility, that serve <15 residences or farms that do not have access to a public water system. Community and individual water systems serve approximately 243 million persons in the United States (91% of the U.S. population) and 24 million (9%), respectively. In addition, millions of persons use noncommunity systems while traveling or working. Each drinking-water system associated with a WBDO is classified as having one of the following deficiencies: 1 = untreated surface water; 2 = untreated groundwater; 3 = treatment deficiency (e.g., temporary interruption of disinfection, chronically inadequate disinfection, and inadequate or no filtration); 4 = distribution system deficiency (e.g., cross-connection, contamination of water mains during construction or repair, and contamination of a storage facility); and 5 = unknown or miscellaneous deficiency (e.g., contaminated bottled water). If more than one deficiency is noted on the report form for an outbreak, only the one most likely to have caused the outbreak is noted. Recreational waters encompass swimming pools, whirlpools, hot tubs, spas, water parks, and naturally occurring fresh and marine surface waters. Although the surveillance system includes whirlpool- and hot tub-associated outbreaks of dermatitis caused by Pseudomonas aeruginosa, it does not include wound infections resulting from waterborne organisms (e.g., Aeromonas species). Classification of Outbreaks The surveillance system classifies WBDOs according to the strength of the evidence implicating water (Table_1). The classification numbers (i.e., I IV) are based on the epidemiologic data provided and the presence or absence of water-quality data on the report form. Epidemiologic data are weighted more heavily than water-quality data. Thus, although some outbreaks without water-quality data were included in this summary, reports of the use of contaminated water were omitted if they did not provide supporting epidemiologic data. The classification numbers are included in the line listings to indicate what data were available. A classification of I means that adequate epidemiologic and water- quality data were reported but does not necessarily imply that the investigation was optimal. Classification numbers of II IV do not necessarily imply that the investigations were flawed; the circumstances of each outbreak differ, and not all outbreaks can or should be rigorously investigated. Outbreaks of pseudomonas dermatitis and single cases of primary amebic meningoencephalitis or of illness resulting from chemical poisoning are not classified according to this scheme. RESULTS 1993-1994 Outbreaks Associated With Drinking Water For the period 1993-1994, 17 states and one territory reported a total of 30 outbreaks associated with drinking water. Eighteen outbreaks were reported for 1993 and 12 for 1994. Thirteen (43.3%) of the 30 outbreak reports were classified as Class I (i.e., adequate epidemiologic and water- quality data were provided), three (10.0%) as Class II, and 10 (33.3%) as Class III; three single cases of lead poisoning and one single case of nitrate poisoning were not classified (13.3%). Outbreaks are listed individually by state (Table_2 and Table_3) and are tabulated by the etiologic agent and type of water system (Table_4) and by the type of deficiency and type of water system (Table_5). The months with the greatest number of outbreaks (five per month each) were March, June, and August (Figure_1). The months of onset are not known for two outbreaks. Seven (23.3%) of the 30 outbreaks were reported by two states, Pennsylvania (four outbreaks) and Minnesota (three). The outbreaks caused an estimated 405,366 persons to become ill: 403,000 persons during one outbreak and 2,366 during the other 29. The median outbreak size was 33.5 persons (range: 1 403,000). An estimated 4,442 persons were hospitalized; 4,419 of these persons had cryptosporidiosis, 22 had bacterial gastroenteritis, and one had acute gastrointestinal illness of unknown etiology (AGI). Seven persons infected with Salmonella serotype Typhimurium died, as did an unknown number of persons who had cryptosporidiosis. Etiologic Agents Twenty-two (73.3%) of the 30 outbreaks were known or suspected to be associated with infectious agents and eight (26.7%) with chemical contaminants. A protozoan parasite (G. lamblia or C. parvum) was identified as the causative agent for 10 outbreaks, representing 33.3% of the 30 outbreaks and 40.0% of the 25 WBDOs for which the etiologic agent was determined. Cryptosporidium. Five outbreaks of cryptosporidiosis, which resulted in illness in an estimated 403,271 persons, occurred in Minnesota (one outbreak), Nevada (one), Washington (two), and Wisconsin (one). The outbreaks occurred in March (one), April (one), August (two), and December (one). Three were associated with community water systems and one each with either a noncommunity or an individual system; three systems used lake water that was filtered and chlorinated, and two used untreated well water. The outbreak of cryptosporidiosis in Milwaukee was the largest documented WBDO in the United States since record keeping began in 1920. An estimated 403,000 persons became ill, of whom 4,400 were hospitalized (11,12). Accurate estimates of the number of deaths are not currently available. The outbreak was associated with water that had been filtered and chlorinated after it was obtained from Lake Michigan. Deterioration in raw-water quality and decreased effectiveness of the coagulation-filtration process led to an increase in the turbidity of treated water ** and to inadequate removal of C. parvum oocysts (11). Although the treated water met all state and federal quality standards that were then in effect, C. parvum oocysts were found in ice blocks that were made during the outbreak period. The original environmental source of the oocysts was not definitively determined. Factors that contributed to the recognition of the Milwaukee outbreak included widespread absenteeism among hospital employees, students, and school teachers; increased numbers of emergency room visits for diarrheal illness; and a citywide shortage of antidiarrheal drugs. The etiologic agent and the waterborne nature of the outbreak were not identified until at least 2 weeks after the onset of the outbreak. Thereafter, a boil-water advisory was issued. The outbreak of cryptosporidiosis in Clark County, Nevada, which includes Las Vegas, lasted approximately 7 months and was first recognized among persons infected with the human immunodeficiency virus (HIV) (13). Two major factors contributing to the detection of the outbreak were that cryptosporidiosis had been a reportable disease in Nevada for several years, and that many persons who have acquired immunodeficiency syndrome live in Clark County. Cryptosporidiosis is more likely to be diagnosed in such persons because they usually have severe symptoms and often have physicians who are knowledgeable about this disease and know how to diagnose it. Testing for C. parvum is not done routinely in most microbiology laboratories. HIV-infected persons who were diagnosed with cryptosporidiosis during the outbreak period had an increased likelihood of dying by the end of the outbreak period but did not have an increased 1-year mortality rate. Data obtained during the investigation of the outbreak indicated that cryptosporidiosis also affected non-HIV-infected persons. However, no estimates of the total number of persons in the general population who had outbreak-related illness are available. Drinking water was not implicated until after the end of the outbreak; therefore, a boil-water advisory was not issued. The Nevada outbreak of cryptosporidiosis was associated with water obtained from Lake Mead that was filtered and chlorinated during treatment. Before and during the outbreak, the water quality was much better than that required by current national standards. For example, in the 3 years before the outbreak, the mean daily turbidity levels for source and treated water had been <0.2 and <0.1 nephelometric turbidity units, respectively, with no marked increases before or during the outbreak. *** Although no C. parvum oocysts were detected in the source or finished water during the outbreak period, presumptive oocysts were found in both types of water and in filter backwash that was sampled thereafter. As noted previously, the outbreak of cryptosporidiosis in Minnesota also was associated with filtered and chlorinated lake water; C. parvum oocysts and G. lamblia cysts were found in lake water but not in finished water. The two outbreaks of cryptosporidiosis in Washington were associated with well water. The first outbreak, which occurred in 1993, was associated with untreated water from a shallow well that was contaminated by surface water. The second outbreak, in 1994, was associated with a damaged irrigation system that allowed treated waste water to flow into a well. For both outbreaks, presumptive C. parvum oocysts were found in the well water. In addition, for one of these outbreaks, fecal coliforms were found; for the other, total coliforms were found. Giardia. The five outbreaks of giardiasis associated with drinking water affected an estimated 385 persons and were reported from New Hampshire (two outbreaks), Pennsylvania (one), South Dakota (one), and Tennessee (one). The outbreaks occurred in January (one), March (one), May (two), and September (one). All were associated with community water systems; three were associated with surface water and two with well water. The two outbreaks of giardiasis in New Hampshire were associated with unfiltered, chlorinated surface water. For one outbreak, water from a reservoir tested positive for G. lamblia. For the other outbreak, finished water was positive for total coliforms. For both outbreaks, some of the cases were geographically clustered close to the sites where the water was chlorinated (i.e., 27.8% of the former and 50.0% of the latter cases were clustered along proximal portions of the main lines of the respective distribution systems). This suggests that, for these cases, the chlorine contact time was inadequate. In Tennessee, a cross-connection between potable and waste-water lines was associated with an outbreak of giardiasis in a correctional facility (14). Potable water was used to cool the seals of a waste-water pump; a fall in pressure in the potable-water system probably caused waste water to flow back into the potable-water line. Subsequently, high levels of G. lamblia cysts were found in tap water (581 cysts/L). Of 424 stool specimens collected from persons in the correctional facility, 110 (25.9%) were positive for G. lamblia and 42 others (9.9%) for Entamoeba histolytica. The outbreak of giardiasis in Pennsylvania was associated with filtered and chlorinated well water that was contaminated with sewage. Tests indicated that G. lamblia and E. coli were present in tap water. The outbreak in South Dakota was attributed to untreated well water contaminated by water from a nearby creek; G. lamblia was found in the well water, and fecal coliforms were found in both well and tap water. Bacteria. Seven outbreaks were caused by bacteria; five of these outbreaks were associated with untreated well water, one with chlorinated well water, and one with bottled water. The outbreak in Missouri that was caused by Salmonella serotype Typhimurium resulted in illness in an estimated total of 625 persons, including 15 persons who were hospitalized and seven who died. The most likely source for the outbreak was the larger of two storage towers, which was inadequately protected from wild-bird droppings. S. Typhimurium was isolated from the sediment of one of the towers, and tap water was positive for fecal coliforms. The 1993 outbreak in Minnesota that was caused by Campylobacter jejuni was associated with untreated well water, which probably was contaminated in a storage tower that had been cleaned the previous month. During the investigation of this outbreak, fecal coliforms were found in water from the storage tower. In Saipan, Northern Mariana Islands, an outbreak caused by non-O1 Vibrio cholerae and associated with contaminated bottled water caused illness in 11 persons, four of whom were hospitalized. Because public tap water in most locales in Saipan is too salty to be drinkable, two commercial bottled-water plants treat municipal water **** with reverse osmosis to supply drinking water to the public. Although the bottles were to have been cleaned by machine or manually with hot water and a chlorine solution, the bottling plants had occasionally been cited for the cursory handling of returned bottles (e.g., for only rinsing them with treated water). During the outbreak period, bottled water tested positive for fecal coliforms. The source of the contamination was not determined. Chemicals. Eight outbreaks of chemical poisoning were reported, three of which were individual cases of lead poisoning. Two of these three cases were detected by a lead-screening program. The three cases were in infants who had blood-lead levels of 15 ug/dL, 37 ug/dL, and 42 ug/dL. CDC's level of concern is 10 ug/dL (15). For all three cases, lead had leached from brass fittings and lead-soldered seams in drinking-water storage tanks (16). The three water systems used highly demineralized drinking water that had intensified the leaching process. The cases are counted separately (Table_2) because it is not known whether lead had leached from the storage tanks because of the way they were manufactured (at least two of the storage tanks were made by the same company) or because of repairs or other changes made to them after they left the manufacturer(s). In the two outbreaks of fluoride poisoning (i.e., in Mississippi and Hawaii), more than half the exposed persons became ill (34 of 62 and 9 of 16, respectively). Symptoms included nausea, diarrhea, abdominal pain, headache, and dizziness. For both outbreaks, the water was accidentally overdosed with fluoride because the fluoridation chemical was siphoned through the pump into the drinking water. This resulted in tap-water levels of fluoride in the Mississippi outbreak of 48-200 mg/L and in Hawaii of 220 mg/L. When levels exceed 10 mg/L, the fluoridation system should be turned off immediately (17). Elevated copper levels in tap water were associated with gastrointestinal illness in at least 43 persons at a hotel. With the exception of one water sample that had a copper level of 156 mg/L, the highest copper level documented for the many other tested samples was 4.7 mg/L (EPA's action level is 1.3 mg/L {18}). The source of copper was the plumbing system within the building. Prolonged stagnation of the water in the pipes contributed to the outbreak. In Indiana, two episodes of nitrate poisoning were associated with eight miscarriages. In the first episode, three women living within 1 mile (1.6 km) of each other reported a total of six miscarriages during 1993 and 1994 (W.F. Grant, personal communication, 1996). Five of the miscarriages occurred in the eighth gestational week, and one occurred in the eleventh. All three women drank water from private wells on their premises; all three wells tapped the same aquifer, which probably was contaminated by a leaking waste pit on a nearby hog farm. Water from the three wells had high nitrate (nitrate-nitrogen or NO subscript 3-N) levels: 19.0 mg/L, 19.2 mg/L, and 26.0 mg/L. EPA's maximum-contaminant level is 10 mg/L (4). Additional testing of well water did not demonstrate elevated levels of volatile or semi-volatile organics, pesticides, heavy metals, or coliform bacteria. All three women delivered full-term babies after switching to nitrate-free sources of drinking water. During the same period, five women who lived in the same geographic area and who had wells with nitrate levels ranging from 1.6 mg/L to 8.4 mg/L (median: 1.7 mg/L) had five normal pregnancies. In the second episode (1994), a woman with five children had two miscarriages within a 6-month period after moving to a new residence in Indiana, which was located about 12 miles (19 km) from the residences of the three women described in the first episode. Both of these pregnancies terminated in their eighth week. The private well at her residence had a nitrate level of 28.7 mg/L and was thought to have been contaminated by the family's septic system. Unidentified Etiologic Agent. No etiologic agent was identified for five (16.7%) of the 30 WBDOs associated with drinking water. The illnesses associated with two of these outbreaks had incubation periods, durations, and symptom complexes that were consistent with viral syndromes. For the four outbreaks for which testing was done, stool specimens were negative for bacterial pathogens. Three outbreaks were associated with untreated well water and the other two with inadequate chlorination of unfiltered well water. For four outbreaks, fecal coliforms were found in water samples. Water-Quality Data Water-quality data (e.g., information as to the presence of coliform bacteria, pathogens, or chemical contaminants) were obtained less than or equal to 1 month after the onset of the outbreak for 28 outbreaks and later for the two episodes of nitrate poisoning. Water samples were tested for coliform bacteria for 21 of the 22 WBDOs that had a known or suspected infectious etiology; the samples were positive for total or fecal coliforms for 16 (76.2%) of the 21 outbreaks. Coliforms were detected for 11 (91.7%) of the 12 outbreaks of bacterial or unknown etiology but for only five (55.6%) of the nine protozoan outbreaks for which testing was done. For these nine outbreaks, coliforms were detected for two of the six systems with chlorinated water but for all three with untreated water. For three of the nine outbreaks, water was positive for C. parvum; for another three, it was positive for G. lamblia. For one (14.3%) of the seven outbreaks with a bacterial etiology, the pathogen (Salmonella) was isolated from the water. Water System and Water Source Fourteen (46.7%) of the 30 WBDOs were associated with community systems, nine (30.0%) with noncommunity systems, and seven (23.3%) with individual systems (Table_4, Table_5, Figure_2). In contrast, for 1991-1992, more of the reported WBDOs were associated with noncommunity (64.3%) than with community systems (26.2%) (2, updated with data from previously unreported outbreaks). During 1993-1994, outbreaks in noncommunity systems were more likely than those in community systems to be associated with untreated water (55.6% versus 14.3%). Eight (88.9%) of the nine outbreaks in the noncommunity systems were associated with well-water sources, as were eight (57.1%) of the 14 community outbreaks. Of the 22 outbreaks with a known or suspected infectious etiology, 16 (72.7%) occurred in systems using well water, and six (27.3%) were associated with a surface-water source. For 11 (68.8%) of the 16 well-water systems associated with the outbreaks, the water was untreated. For four (25.0%) of these 16 systems, all of which used chlorine for disinfection, inadequate or interrupted disinfection was the deficiency identified or suspected (e.g., coliforms, which are chlorine sensitive, were present in tap water). For one of these four systems, deficiencies in filtration also were suspected. For one (6.3%) of the 16 systems (i.e., in Saipan), no treatment deficiency was noted. Six (85.7%) of the seven outbreaks associated with a lake or reservoir (i.e., surface-water sources) were caused by C. parvum (three outbreaks) or G. lamblia (three). Each of the six systems associated with these six outbreaks provided chlorination, and four also provided filtration. In the filtered systems, deficiencies in the distribution system were identified for one outbreak, inadequate filtration was identified for one, and no deficiencies were identified for two. In the two unfiltered systems associated with outbreaks of giardiasis, an inadequate contact time with the disinfectant was suspected. 1993-1994 Outbreaks Associated With Recreational Water For the period 1993-1994, 14 states reported a total of 26 outbreaks associated with recreational water (Table_6, Table_7). Fourteen outbreaks were reported for 1993 and 12 for 1994. Three of the 14 states submitted 14 (53.8%) of the 26 reports: Minnesota (seven outbreaks), Wisconsin (four), and New Jersey (three). For the 2-year reporting period, the months with the greatest number of outbreaks were July (six) and August (five) (Figure_1). All 14 outbreaks of gastrointestinal illness occurred during the months of April through September. Eight of the 11 outbreaks of dermatitis (i.e., rash or folliculitis), which were associated with hot tubs, whirlpools, and swimming pools, occurred during the relatively colder months of November through March. The 26 outbreaks caused illness in an estimated 1,714 persons. The median outbreak size was 32.5 persons (range: 1-418). The types of reported illnesses included gastroenteritis (14 outbreaks), dermatitis (11), and meningoencephalitis (one). Fifteen persons reportedly were hospitalized. The one child who had amebic meningoencephalitis died. The etiologic agent was identified for all 14 outbreaks of swimming- associated gastroenteritis (Table_6, Figure_3). Ten (71.4%) of the 14 outbreaks were caused by the protozoan parasites C. parvum (six outbreaks) and G. lamblia (four). Five of the six C. parvum outbreaks were associated with motel (three) or community (two) swimming pools that were filtered and chlorinated (19,20). For four of these five outbreaks, no treatment deficiencies were identified in the pools; for the other, a malfunctioning filter was found. For two of these five, water was tested and was negative for C. parvum. The tested water, however, had been obtained approximately 2 weeks after the onset of the outbreaks; for one of the outbreaks, water samples were not collected until after the pool had been hyperchlorinated. The first reported U.S. outbreak of cryptosporidiosis linked to the recreational use of lake water occurred in New Jersey during the summer (1994) and lasted >4 weeks. In this outbreak, C. parvum was detected in lake-water samples obtained 5 weeks after the end of the outbreak period. The four G. lamblia outbreaks were related to unintentional ingestion of water from two lakes (two outbreaks), a river (one), and a community swimming pool and wading pool (one). In the latter outbreak, which lasted >3 months, intermittent breakdowns of the swimming pool's filter and lack of water filtration in the wading pool were noted. Water samples from the two lakes that were associated with outbreaks were negative for G. lamblia. However, samples from one lake were obtained 2 weeks after the onset of the outbreak, and samples from the other lake were obtained 4 weeks after the outbreak began. Four outbreaks of gastroenteritis were attributed to bacterial pathogens and were associated with swimming in lakes. S. sonnei was implicated for two of these outbreaks, S. flexneri for one, and E. coli O157:H7 for one. The latter was the second reported outbreak in the United States of E. coli O157:H7 associated with recreational water (2). For the outbreak in New Jersey that was caused by S. sonnei, water samples obtained 4 weeks after the onset of the outbreak were negative for the etiologic agent. For the outbreak in Ohio that was caused by S. sonnei, water samples demonstrated high fecal-coliform counts. The presence of many bathers and poor water exchange were contributing factors for at least one of the four outbreaks. For another, lake water used in a shower frequented by toddlers was circulated back into the lake; high fecal-coliform counts were found in water from the shower's drain basin. An estimated 247 persons were affected in nine outbreaks of dermatitis that were associated with hot tubs, whirlpools, or swimming pools. These nine outbreaks had a known or suspected infectious etiology (Table_7). P. aeruginosa was confirmed as the etiologic agent in five of the outbreaks and was suspected (based on the clinical syndrome) in the other four. In six of these nine investigations, water samples demonstrated low chlorine levels, the presence of Pseudomonas, or both. The two outbreaks of probable chemical dermatitis affected an estimated 29 persons. These outbreaks were associated with incorrect dosing of acidifying chemicals used for correcting the pH of the swimming-pool water or with addition of unspecified chemicals for removing excess chloramines. One case of fatal primary amebic meningoencephalitis was associated with swimming in both a waste-water holding pond and in the Rio Grande River. The etiologic agent, Naegleria fowleri, was found in the child's cerebrospinal fluid and in both the pond and the river. Previously Unreported Outbreaks Reports of 12 previously unpublished WBDOs from 1992 also were received (Table_8, Table_9). For the eight outbreaks associated with drinking water, an estimated 220 persons were reported ill, six of whom were hospitalized. One outbreak caused by S. sonnei was associated with untreated water from a noncommunity well and another with an individual cistern. An outbreak caused by hepatitis A virus was associated with an untreated supply of well water (noncommunity) and is the only outbreak in this surveillance summary in which a viral pathogen was identified (21). Two outbreaks of AGI were associated with noncommunity well-water systems; for both, coliforms were found in tap water. For one of these outbreaks, the clinical features were consistent with a viral syndrome. Three outbreaks of gastrointestinal illness were associated with newly installed, copper plumbing systems. For these three outbreaks, tap water had elevated levels of copper (22). The four WBDOs associated with recreational water caused an estimated total of 203 persons to become ill, one of whom was hospitalized. Two outbreaks of gastroenteritis caused by S. sonnei were associated with swimming in lakes; high total coliform counts were found in the swimming areas of both lakes. In the New Jersey outbreak caused by S. sonnei, a bulkhead had been placed in the lake, which essentially created a swimming pool that had insufficient exchange of water. In a third report, no etiologic agent was identified for an outbreak of AGI associated with swimming in a pool at a water park; the clinical features were consistent with a viral syndrome. Before the outbreak, muddy run-off from a landscaping project had caused the pool's filter to break down. In a fourth report, a clinical syndrome consistent with schistosomal dermatitis (i.e., swimmer's itch) was noted for a New Jersey outbreak associated with swimming in a lake. Outbreaks Not Classified as Waterborne-Disease Outbreaks Outbreaks attributed to drinking water that was contaminated at its point of use rather than at its source or in its distribution system traditionally are not classified as WBDOs. Three such outbreaks of AGI were reported; they caused illness in a total of 376 persons. The outbreaks were associated with the contamination of two water jugs, a water hose, or ice from an ice chest. Data relating to 10 other possible WBDOs were not included. One of these outbreaks was more likely to have been foodborne than waterborne, and one was a report of two cases of leptospirosis among persons exposed to flood waters during clean-up operations (23). For eight of these possible WBDOs, inadequate data were provided (i.e., the outbreaks did not meet the criteria for Classes I-IV). DISCUSSION General Considerations About Surveillance Data for Waterborne-Disease Outbreaks The surveillance data, which identify the types of water systems, their deficiencies, and the respective etiologic agents associated with the outbreaks, are useful for evaluating the adequacy of current technologies for providing safe drinking and recreational water. However, the data in this surveillance summary are subject to at least one important limitation: they probably do not reflect the true incidence of WBDOs or the relative incidence of outbreaks caused by various etiologic agents. Not all WBDOs may be recognized, investigated, and/or reported to CDC or EPA, and the extent to which WBDOs are unrecognized and underreported is unknown. The likelihood that individual cases of illness will be epidemiologically linked and associated with water varies considerably among locales and is dependent on factors such as public awareness, physician interest, availability of laboratory-testing facilities, and surveillance activities of state and local health and environmental agencies. Therefore, the states that report the most outbreaks may not be those in which the most outbreaks occur. Recognition of WBDOs also is dependent on certain outbreak characteristics; outbreaks involving serious illness are most likely to receive the attention of health authorities. In cities, large outbreaks are more likely to be recognized than sporadic cases or small outbreaks in which ill persons may consult different physicians. Outbreaks associated with community water systems are more likely to be recognized than those associated with noncommunity systems because the latter serve nonresidential areas and transient populations. Outbreaks associated with individual systems are the most likely to be underreported because they generally involve few persons. Outbreaks of acute diseases, particularly those characterized by a short incubation period, are more readily identified than those associated with disease from chronic, low-level exposure to an agent (e.g., a chemical). The identification of the etiologic agent of a WBDO is dependent on the timely recognition of the outbreak so that appropriate clinical and environmental samples can be obtained. The interests and expertise of investigators and the routine practices of local laboratories also influence whether the etiologic agent is identified. For example, diarrheal stool specimens generally are examined for bacterial pathogens but not for viruses. In most laboratories, testing for C. parvum is done only on request and is not included in routine stool examinations for ova and parasites. The water-quality data that are collected vary widely among outbreak investigations, depending on such factors as available fiscal, investigative, and laboratory resources. Furthermore, a few large outbreaks may substantially alter the relative proportion of cases of waterborne disease attributed to a particular agent. The number of reported cases is generally an approximate figure, and the method and accuracy of the approximation vary among outbreaks. 1993-1994 Outbreaks Associated With Drinking Water The numbers of outbreaks reported for 1993 (18) and 1994 (12) are comparable with those reported for recent years (except for 1992). With the addition of previously unreported outbreaks, 27 WBDOs have been reported for 1992 (the highest number since 1984), 15 for 1991, 14 for 1990, and 13 for 1989 (2,3). WBDO reports peaked during 1979-1983 (Figure_4, Figure_5). The increase and subsequent decrease in the number of reports may reflect, at least in part, changes in surveillance activities rather than a deterioration or an improvement in water systems (24). Having only 16.7% of the WBDOs reported for 1993-1994 of unknown etiology (i.e., AGI outbreaks) is unprecedented for the last 24 years (Figure_4). All AGI outbreaks occurred in noncommunity systems, which underscores the difficulty of investigating outbreaks affecting the transient populations that typically use the water from these systems. The massive outbreak in Milwaukee highlights the need for surveillance methods that facilitate the early detection of outbreaks and the prompt institution of preventive measures (e.g., boil-water advisories). This outbreak also demonstrates that WBDOs are not restricted to small communities, in which WBDOs are often attributed to a lack of proper equipment or inadequately trained operators. As in previous years, protozoan parasites were the most frequently identified etiologic agents; for the period 1993-1994, C. parvum and G. lamblia were each identified for five outbreaks. The increased number of reported outbreaks of cryptosporidiosis in recent years may reflect the growing awareness that C. parvum can be transmitted by the waterborne route. For the period 1976-1994 as a whole, G. lamblia was the most frequently identified etiologic agent for outbreaks associated with drinking water. The occurrence of the two outbreaks of giardiasis in New Hampshire (1994) underscores the importance of requiring water systems that use unfiltered surface water to provide an adequate chlorine concentration and contact time (as specified by the SWTR) to inactivate relatively chlorine-resistant organisms such as G. lamblia (25). C. parvum oocysts have been detected in 65%-97% of the surface-water supplies throughout the United States that have been tested recently (26-28). Because C. parvum is highly resistant to the chemical disinfectants typically used to treat drinking water, ***** the physical removal of the parasite by filtration (e.g., rapid-granular, slow sand, or membrane filtration) is an important component of the municipal water- treatment process. Rapid-granular filtration with sand or some other media, alone or in combination, is used by many municipalities as part of their conventional treatment of surface water. To be effective in removing oocysts, rapid-granular filtration must be preceded by chemical coagulation and the treatment must be optimized to remove particles. Even when done appropriately, rapid-granular filtration cannot guarantee the removal of all the oocysts. Some major cities use surface-water sources but do not filter their water (e.g., Boston, New York City, Portland, San Francisco, and Seattle). They are, however, either proceeding with plans to filter their water, or, based on criteria set forth in the SWTR, are not required to filter it. From 1984 through 1992, all reported U.S. outbreaks of cryptosporidiosis associated with drinking water occurred in communities whose water utilities met the then-current state and federal standards for acceptable drinking-water quality. The surface-water supplies that were implicated in these outbreaks had been filtered (2,30-32). In 1993 and 1994, the water systems implicated in the three surface water-associated outbreaks of cryptosporidiosis also met federal and state standards. The occurrence of such outbreaks indicates that compliance with EPA's water- treatment standards (e.g., for turbidity and coliform counts) did not adequately protect against waterborne cryptosporidiosis. For example, C. parvum can be present in chlorinated water despite the absence of coliforms. That coliforms were detected for only 55.6% of the protozoan outbreaks (vs. for 91.6% of the outbreaks of bacterial or unknown etiology) suggests that the use of coliforms as indicators of protozoan contamination is not reliable. Efforts to reduce the risk for WBDOs of cryptosporidiosis should focus on the protection of source water, the removal of oocysts by filtration, and research to identify effective and affordable disinfection procedures. Decreased filtration effectiveness, combined with deterioration in raw-water quality, contributed to the outbreak of cryptosporidiosis in Milwaukee. Optimal filtration requires frequent, if not continuous, monitoring of the water's turbidity both before filtration (i.e., after coagulation, flocculation, and/or settling) and after filtration. In addition, the quality of the source water should be frequently assessed and the amount of added coagulant appropriately adjusted. More stringent EPA standards for acceptable turbidity values, which serve as an index of the effectiveness of filtration, have become effective in all states since the Milwaukee outbreak (6). The water utility in Milwaukee, at the time of the outbreak, would not have met these new standards. Recent investigations, however, have demonstrated small numbers of C. parvum oocysts in fully treated (i.e., filtered and disinfected) water from 27%-54% of the municipal treatment plants studied, most of which met the new standards (33,34). A workshop sponsored by CDC and EPA on waterborne cryptosporidiosis was held in Atlanta in September 1994 ****** to discuss newly proposed EPA regulations and the health risks associated with drinking tap water contaminated with small numbers of oocysts (35,36). Those attending the workshop concluded that the magnitude of the risk posed by these oocysts could not yet be assessed because of several factors, including uncertainty about the viability or infectivity of the oocysts, the infectious dose for immunocompetent and immunocompromised persons, and the possibility of strain variability in the infectious dose and in the ability to cause disease. The results of a study that used a C. parvum strain derived from calves suggested that the infectious dose of oocysts for healthy persons is small (i.e., a median of 132 oocysts) (37). Information based on mathematical modeling suggests that some persons might become infected if exposed to even one oocyst (38). Until the magnitude of the risk for waterborne cryptosporidiosis is better characterized or until effective procedures for removing oocysts from water are identified, severely immunocompromised persons should be provided with educational material about the potential risk for waterborne disease and the strategies for prevention (39,40). During 1993-1994, five of the seven outbreaks caused by bacterial agents occurred in systems that used untreated well water. During 1991- 1992, all three outbreaks caused by bacteria and the two caused by hepatitis A virus were associated with untreated well or cistern water. Adequate, continuous disinfection of groundwater used for drinking water should reduce the occurrence of WBDOs, particularly for small systems in which intermittent contamination of wells and springs is difficult to detect or prevent. In addition, wells and springs should be protected from sources of contamination such as surface runoff, septic-tank drainage, and sewage discharges. The two outbreaks in Minnesota and Missouri in 1993 associated with contaminated storage towers are reminders that stored water should be protected from contamination (e.g., by feces from birds and small mammals). They also demonstrate that, to prevent flushing of sediment or stagnant water from storage towers into the distribution system, caution should be exercised when such towers are cleaned. The three cases of lead poisoning in infants were included in this summary because blood-lead levels at least as low as 10 ug/dL are associated with adverse health effects (15,41). Lead is particularly harmful to the developing brain and nervous system of fetuses and young children; elevated blood-lead levels are associated with decreased intelligence and impaired neurobehavioral development. The three cases of lead poisoning and the one outbreak of copper poisoning underscore the importance of using appropriate materials in household plumbing systems. The two outbreaks of fluoride poisoning highlight the need for appropriate design (e.g., the incorporation of antisiphon devices) and maintenance of water-fluoridation systems, as well as for monitoring fluoride levels in water (42). The two episodes of an association between miscarriages and elevated nitrate levels in drinking water are the first such episodes to be reported to the WBDO surveillance system. Although ingestion of water with high nitrate levels is known to cause methemoglobinemia in infants (43), little information is currently available about the effects of nitrate on fetal development in humans. A recent, geographically based survey of 5,536 private wells serving individual households in nine Midwestern states demonstrated that 13% of the wells had nitrate levels in excess of EPA's maximum-contaminant level (CDC, unpublished data). The two episodes reported in this surveillance summary raise questions about the importance of nitrate as a cause of miscarriages and the need to provide both educational information regarding nitrate in drinking water and affordable access to water-testing services. During 1993-1994, only eight outbreaks/cases of chemical poisoning were reported to CDC. For 1993 alone, however, the American Association of Poison Control Centers reported 791 nitrate and nitrite poisonings, 887 copper poisonings, and 5,675 poisonings associated with swimming pools (44). Although their report does not provide information as to whether these poisonings were waterborne, some of the nitrate and many of the copper poisonings may have been associated with drinking water. Several reasons may help explain why waterborne chemical poisonings are rarely reported to CDC: a) most poisonings of this nature (e.g., those associated with the leaching of lead or copper from plumbing systems) probably occur in private residences, affect relatively few persons and, thus, might not come to the attention of public health officials; b) exposure to chemicals via drinking water may cause illness that is difficult to attribute to chemical intoxication, or it may cause nonspecific symptoms that are difficult to link to a specific agent; and c) the mechanisms for detecting waterborne chemical poisonings and reporting them to the WBDO surveillance system are not as well established as they are for WBDOs caused by infectious agents. CDC, in collaboration with EPA and the U.S. Geological Survey, is attempting to establish mechanisms for triggering epidemiologic investigations of suspected acute exposures to pollutants and for evaluating the deleterious effects of short- and long-term exposures to hazardous substances. Currently, water at treatment plants is monitored for various organic and inorganic substances, and household tap water is monitored for lead and copper. Future monitoring efforts should be tailored to improve a) the sensitivity of surveillance activities, b) the detection of associations between environmental releases or exposure incidents and individual health events, and c) the assessment of the public health burden associated with water-related chemical exposures. Although definitive conclusions cannot be reached about some of the WBDOs reported for 1993-1994, most of them probably could have been prevented by using available water-treatment technologies and standard operating/monitoring procedures. Measures that could have been used to prevent some of the outbreaks of chemical etiology include the application of existing knowledge about how to avoid the siphonage of fluoridation chemicals into water, the use of appropriate materials in water pipes and storage tanks, and the education of the public regarding the effects of corrosive water on plumbing materials. Most of the WBDOs with a known or suspected infectious etiology could have been prevented if the water utilities had met current regulations and followed appropriate treatment practices and operational procedures, source and treated water had been adequately monitored, and periodic sanitary surveys had been conducted to identify and correct sources of contamination. However, the outbreak of cryptosporidiosis in Nevada demonstrates that WBDOs sometimes can occur despite excellent source-water quality and state-of-the-art water treatment. 1993-1994 Outbreaks Associated With Recreational Water The most frequently reported WBDOs caused by exposure to recreational water were outbreaks of gastroenteritis. Swimming and other recreational activities in which the unintentional ingestion of water can occur are known to increase the risk for gastrointestinal illness, even in nonoutbreak settings (45,46). The number of outbreaks of gastroenteritis for 1993-1994 (i.e., 14) was similar to those previously reported (i.e., 14 for 1991-1992 and 13 for 1989-1990). For 1993-1994, however, 10 (71.4%) of the 14 gastroenteritis outbreaks were attributed to protozoan parasites, compared with six (42.9%) of 14 for 1991-1992, none of 13 for 1989-1990, and two (18.2%) of 11 for 1987-1988. Previous reports of cryptosporidiosis and giardiasis attributed to a waterborne source may have led to an increased awareness that these diseases can result from waterborne transmission. For example, four outbreaks of cryptosporidiosis associated with the use of recreational water were identified in Wisconsin in 1993 after the large drinking-water outbreak in Milwaukee. The failure to identify deficiencies in filtration or chlorination for four of the six outbreaks of pool-associated gastroenteritis caused by protozoa indicates that current methods of water treatment may not always ensure protection against protozoan infections in these settings. The inactivation of G. lamblia cysts by the routine chlorination of swimming pools may require >15 minutes, depending on such factors as temperature, pH, and chlorine concentration. Under normal operating conditions for pools, at least several days would be needed to inactivate C. parvum oocysts. Several types of filters are used in swimming pools. Their effectiveness for removing oocysts is not known; and their filtration rates are generally slow, requiring up to 6 hours for a complete turnover of pool water (47). During the outbreak of recreational lake water-associated cryptosporidiosis in New Jersey, water temperatures ranged from 86 F to 95 F (30 C to 35 C). Preliminary data, based on an ongoing study funded by the American Water Works Association Research Foundation, indicate that the number of viable oocysts, determined by excystation, in surface water decreases by 90%-99% after 28 days at water temperatures of 86 F (30 C) (M.W. LeChevallier, personal communication, 1995). Other factors, such as the effects of sunlight on oocysts in shallow bodies of water and the density and composition of heterotrophic microflora, might further decrease the survival time of oocysts in water. Although the outbreak, which lasted >4 weeks, may have resulted from a single contamination event, the long period of transmission also may have been caused by the reintroduction of C. parvum into the lake water by infected persons. Outbreaks of swimming-associated shigellosis continue to occur. The probable source of the pathogen for the three outbreaks in 1993-1994, as for previous outbreaks, was fecal contamination of lake water by persons in the water. In 1994, the second-ever reported outbreak that was caused by E. coli O157:H7 and associated with exposure to recreational water occurred. E. coli O157:H7, like Shigella spp., apparently has a low infectious dose (48,49). Thus, infection may be acquired without swallowing large volumes of water. EPA has published criteria for evaluating the quality of both marine and fresh water used for recreation (50,51). Microbial monitoring has been recommended for recreational areas potentially contaminated by sewage. However, the utility of routinely monitoring untreated water (e.g., lakes) for fecal contamination caused by bathers has not been established. Efforts at prevention have focused on providing adequate toilet and diaper-changing facilities at recreational areas and limiting the number of bathers per unit area. An additional, important measure, although difficult to enforce, is to prevent persons (especially young, nontoilet-trained children) from entering recreational waters if they are either experiencing or convalescing from a diarrheal illness. For the period 1993-1994, most of the reported outbreaks of dermatitis associated with hot tubs, whirlpools, and swimming pools were directly related to inadequate operation and maintenance procedures. Outbreaks of pseudomonas dermatitis associated with hot tubs are preventable if water is maintained at a pH of 7.2-7.8 with free, residual chlorine levels in the range of 2.0-5.0 mg/L (52). Similarly, outbreaks of pseudomonas dermatitis associated with swimming pools are preventable if the pH and chlorine levels are maintained at 7.2-8.2 and 0.4-1.0 mg/L, respectively (47). A person's susceptibility and immersion time, along with the number of bathers per unit area, also may influence the risk for infection (53). The one death associated with the use of recreational water was caused by primary amebic meningoencephalitis, a rarely reported disease in the United States. N. fowleri infections are generally acquired during the summer months, when the temperature of fresh water is favorable for the multiplication of the organism (54,55). CONCLUSIONS Information from the nationwide surveillance of WBDOs is used to characterize the epidemiology of waterborne diseases in the United States. Data about the types of water systems and deficiencies associated with outbreaks are needed to evaluate the adequacy of current regulations for water treatment and monitoring of water quality. The identification of the etiologic agents of outbreaks is particularly critical because agents newly associated with WBDOs may require new methods of control. For agents that are recognized as important waterborne pathogens, rapid recognition and control of WBDOs are facilitated by surveillance at the local and state level. Close communication between local health departments and water utilities is crucial. For example, if epidemiologic evidence suggests the possibility of waterborne transmission, water utilities should be contacted promptly and asked about such factors as recent treatment deficiencies and changes in source-water quality. Similarly, local policies should be developed that specify the thresholds for reporting various water-quality data to health departments. Timely water testing and environmental investigations can facilitate the identification of an outbreak's etiologic agent and the correctable source(s) of water contamination, as well as establish whether control measures (e.g., boil- water advisories) are indicated. Means of improving the surveillance system for WBDOs should be explored. The review of information that has been gathered through other mechanisms (e.g., reports to poison-control centers, issuances of boil- water advisories, and computerized data on water quality) may facilitate the detection of more WBDOs. Special epidemiologic studies may be needed that supplement the findings of this surveillance system by addressing such issues as the public health importance of newly identified agents of waterborne disease and the effectiveness of prevention strategies in nonoutbreak settings. State health departments can request epidemiologic assistance from CDC for the investigation of WBDOs. In addition, CDC and EPA can be consulted about the engineering and environmental aspects of water treatment and about collecting large-volume water samples to identify pathogenic viruses and parasites. Additional information is available from EPA's Safe Drinking Water Hotline (telephone {800} 426-4791; e-mail sdwa@epamail.epa.gov), CDC's Cryptosporidiosis Information Line of the Parasitic Diseases Information Line (voice-fax telephone system {404} 330-1242), and CDC/National Center for Infectious Diseases' home page on the Internet World Wide Web (http://www.cdc.gov/ncidod/diseases/crypto/crypto.htm). Waterborne-disease outbreaks should be reported to CDC's Division of Parasitic Diseases; telephone (770) 488-7760. Acknowledgments The authors thank the state waterborne-disease surveillance coordinators; the state epidemiologists; the state drinking-water administrators; the Office of Ground Water and Drinking Water, U.S. Environmental Protection Agency; the Division of Bacterial and Mycotic Diseases, NCID, CDC; and the Division of Environmental Hazards and Health Effects, NCEH, CDC, for contributing to the waterborne-disease surveillance summary. References
Glossary In this glossary, italicized terms that are not names of microorganisms are defined elsewhere in the glossary. Action level: A specified concentration of a contaminant in water; if this concentration is reached or exceeded, certain actions (e.g., further treatment and monitoring) must be taken to comply with a drinking-water regulation. Boil-water advisory: A statement to the public advising persons to boil tap water before drinking it. Cistern: A storage tank for collected rain water. Class: Refer to the Classification of Outbreaks section in the text and to Table_1 for a comprehensive definition. Coagulation: The process of adding chemicals to water to destabilize charges on naturally occurring particles to facilitate their subsequent aggregation and removal by flocculation and/or filtration. Coliforms: A group of gram-negative, rod-shaped, nonspore-forming aerobic and facultative anaerobic bacteria that ferment lactose, with acid and gas formation within 48 hours at 95 F (35 C). These bacteria are commonly found in soil and in the gut and feces of warm-blooded animals. Their presence in water may indicate contamination with human and/or animal feces. Community water system: A public water system that serves year-round residents of a community, subdivision, or mobile-home park that has greater than or equal to 15 service connections or an average of greater than or equal to 25 residents. Contact time: The length of time water is exposed to a disinfectant (e.g., chlorine contact time). Cross-connection: Any actual or potential connection between a drinking- water supply and a possible source of contamination or pollution (e.g., a waste-water line). Cyst: The infectious stage of Giardia lamblia and some other protozoan parasites that has a protective wall, which facilitates survival in water and other environments. Disinfection by-products: Chemicals formed in water through reactions between organic matter and disinfectants. Distribution system: Water pipes, storage reservoirs, tanks, and other means used to deliver drinking water to consumers or to store it before delivery. Excystation: The release of the internal (i.e., encysted) contents (e.g., trophozoites or sporozoites) from cysts or oocysts. Fecal coliforms: A subset of the coliform group of bacteria that includes such bacteria as Escherichia coli that produce gas from lactose at 112.1+/-0.36 F (44.5+/-0.2 C) and whose presence in water indicates contamination with human and/or animal feces. Filter backwash: The water containing the material obtained by reversing the flow of water through a filter to dislodge the particles that have been retained on it. Filtration: The process of removing suspended particles from water by passing it through one or more permeable membranes or media of small diameter (e.g., sand, anthracite, or diatomaceus earth). Finished water: The water (i.e., drinking water) delivered to the distribution system after treatment, if any. Flocculation: The water-treatment process after coagulation that uses gentle stirring to cause suspended particles to form larger, aggregated masses (floc). The aggregates are removed from the water by a separation process (e.g., sedimentation, flotation, or filtration). Free, residual chlorine level: The concentration of chlorine in water that is not combined with other constituents and thus serves as an effective disinfectant. Groundwater system: A system that uses water extracted from the ground (i.e., a well or spring). Heterotrophic microflora: Microorganisms that utilize organic material for energy and growth. Indicator organism: A microorganism that indicates, by its presence in water, possible or actual contamination by fecal material. Individual water system: A small water system, not owned or operated by a water utility, that serves <15 residences or farms that do not have access to a public water system. Maximum-contaminant level: The maximum permissible concentration (level) of a contaminant in water supplied to any user of a public water system. Nephelometric turbidity units: The units in which the turbidity of a sample of water is measured when the degree to which light is scattered is assessed with a nephelometric turbidimeter. Noncommunity water system: A public water system that a) serves an institution, industry, camp, park, hotel, or business that is used by the public for greater than or equal to 60 days per year; b) has greater than or equal to 15 service connections or serves an average of greater than or equal to 25 persons; and c) is not a community water system. Oocyst: The infectious stage of Cryptosporidium parvum and some other coccidian parasites that has a protective wall, which facilitates survival in water and other environments. Public water system: A system, classified as either a community or a noncommunity water system, that provides piped water to the public for human consumption and is regulated under the Safe Drinking Water Act. Raw water: Untreated water. Reverse osmosis: A filtration process that removes dissolved salts and metallic ions from water by forcing it through a semipermeable membrane. This process is also highly effective in removing microbes from water. Siphonage: A reversal of the normal flow of water or other liquid caused by a negative-pressure gradient (e.g., within a water system). Source water: Untreated water (i.e., raw water) used to produce drinking water. Surface water: The water in lakes, rivers, reservoirs, and oceans. Total coliforms: Nonfecal and fecal coliforms. Turbidity: The quality (e.g., of water) of having suspended matter (e.g., clay, silt, or plankton), which results in loss of clarity or transparency. Untreated water: Refer to raw water. Water utility: A water provider that distributes drinking water to a community through a network of pipes. Watershed: An area from which water drains to a single point; in a natural basin, the area contributing flow (i.e., water) to a given place or a given point on a stream. Watershed-control program: A program to protect a watershed from sources of contamination or pollution. State and Territorial Epidemiologists and Laboratory Directors State and Territorial Epidemiologists and Laboratory Directors are acknowledged for their contributions to CDC Surveillance Summaries. The epidemiologists listed below were in the positions shown as of February 1996, and the laboratory directors listed below were in the positions shown as of February 1996. State/Territory Epidemiologist Laboratory Director Alabama John P. Lofgren, MD William J. Callan, PhD Alaska John P. Middaugh, MD Gregory V. Hayes, DrPH Arizona Norman J. Petersen, SM (Acting) Barbara J. Erickson, PhD Arkansas Thomas C. McChesney, DVM Michael G. Foreman California Stephen H. Waterman, MD, MPH Michael G. Volz, PhD Colorado Richard E. Hoffman, MD, MPH Ronald L. Cada, DrPH Connecticut James L. Hadler, MD, MPH Sanders F. Hawkins, PhD Delaware A. LeRoy Hathcock, PhD Mahadeo P. Verma, PhD District of Martin E. Levy, MD, MPH James B. Thomas, ScD Columbia Florida Richard S. Hopkins, MD, MSPH E. Charles Hartwig, ScD Georgia Kathleen E. Toomey, MD, MPH Elizabeth A. Franko, DrPH Hawaii Richard L. Vogt, MD, MPH Vernon K. Miyamoto, PhD Idaho Jesse F. Greenblatt, MD, MPH Richard H. Hudson, PhD Illinois Byron J. Francis, MD, MPH David F. Carpenter, PhD Indiana Gregory K. Steele, DrPH, MPH David E. Nauth (Acting) Iowa M. Patricia Quinlisk, MD, MPH Mary J. R. Gilchrist, PhD Kansas Gianfranco Pezzino, MD, MPH Roger H. Carlson, PhD Kentucky Reginald Finger, MD, MPH Thomas E. Maxson, DrPH Louisiana Louise McFarland, DrPH Henry B. Bradford, Jr, PhD Maine Kathleen F. Gensheimer, MD, MPH John A. Krueger (Acting) Maryland Diane M. Dwyer, MD, MPH J. Mehsen Joseph, PhD Massachusetts Alfred DeMaria, Jr, MD Ralph J. Timperi, MPH Michigan Kenneth R. Wilcox, Jr, MD, DrPH Robert Martin, DrPH Minnesota Michael T. Osterholm, PhD, MPH Pauline Bouchard, JD, MPH Mississippi Mary Currier, MD, MPH Joe O. Graves, PhD Missouri H. Denny Donnell, Jr, MD, MPH Eric C. Blank, DrPH Montana Todd A. Damrow, PhD, MPH Douglas O. Abbott, PhD Nebraska Thomas J. Safranek, MD John D. Blosser Nevada Randall L. Todd, DrPH Arthur F. DiSalvo, MD New Hampshire M. Geoffrey Smith, MD, MPH Veronica C. Malmberg, MSN New Jersey Lyn Finelli, DrPH (Acting) Marion Pierce New Mexico C. Mack Sewell, DrPH, MS Loris W. Hughes, PhD New York City Benjamin A. Mojica, MD, MPH Stanley Reimer New York State Dale L. Morse, MD, MS Ann Wiley, PhD North Carolina J. Michael Moser, MD, MPH Lou F. Turner, DrPH North Dakota Larry A. Shireley, MS, MPH James D. Anders, MPH Ohio Thomas J. Halpin, MD, MPH Kathleen L. Meckstroth, DrPH Oklahoma J. Michael Crutcher, MD, MPH Garry L. McKee, PhD (Acting) Oregon David W. Fleming, MD Michael R. Skeels, PhD, MPH Pennsylvania James T. Rankin, Jr, DVM, Bruce Kieger, DrPH PhD, MPH Rhode Island Utpala Bandy, MD, MPH Walter S. Combs, PhD South Carolina James J. Gibson, MD, MPH Harold Dowda, PhD South Dakota Susan E. Lance, DVM, PhD, MPH Richard S. Steece, PhD Tennessee William L. Moore, MD Michael W. Kimberly, DrPH Texas Diane M. Simpson, MD, PhD David L. Maserang, PhD Utah Craig R. Nichols, MPA Charles D. Brokopp, DrPH Vermont Vacant Burton W. Wilke, Jr, PhD Virginia Grayson B. Miller, Jr, MD, MPH James L. Pearson, DrPH Washington Paul Stehr-Green, DrPH, MPH Jon M. Counts, DrPH West Virginia Loretta E. Haddy, MA, MS Frank W. Lambert, Jr, DrPH Wisconsin Jeffrey P. Davis, MD Ronald H. Laessig, PhD Wyoming Gayle L. Miller, DVM, MPH Roy J. Almeida, DrPH American Samoa Edgar C. Reid, MO, DSM, MPH -- Federated Vacant -- States of Micronesia Guam Robert L. Haddock, DVM, MPH Florencia Nocon (Acting) Marshall Tom D. Kijner -- Islands Northern Jose L. Chong, MD Isamu J. Abraham, DrPH Mariana Islands Palau Jill McCready, MS, MPH -- Puerto Rico Carmen C. Deseda, MD, MPH Jose Luis Miranda Arroyo, MD Virgin Islands Donna M. Green, MD Norbert Mantor, PhD
** Reported values were as high as 1.7 nephelometric turbidity units; for the previous 10 years, the recorded values had never exceeded 0.4 (11). At the time of the outbreak, national standards for treated water required that 95% of all daily turbidity measurements in a month not exceed 1.0 nephelometric turbidity unit (6). *** Current national standards for treated water require that 95% of all daily turbidity measurements in a month not exceed 0.5 nephelometric turbidity units (6). **** The municipal water supply uses several dozen wells, some of which tested positive for fecal coliforms. ***** For example, at 77 F (25 C) and pH 7, the chlorine contact time to inactivate 99% of C. parvum oocysts is 7,200 (mg/L) x min (29). ****** The workshop was attended by more than 300 persons from 26 organizations (e.g., the water industry; state and local health departments; AIDS interest groups; and the U.S. Department of Agriculture, the Food and Drug Administration, and other federal agencies). Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Classification of investigations of waterborne-disease outbreaks -- United States
============================================================================================================
Class * Epidemiologic data Water-quality data
----------------------------------------------------------------------------------------------------------
I Adequate + Provided and adequate
a) Data were provided about exposed Could be historical information or
and unexposed persons; and laboratory data (e.g., the history that a
b) the relative risk or odds ratio was >=2, chlorinator malfunctioned or a water
or the p-value was <=0.05. main broke, no detectable free-chlorine
residual, or the presence of coliforms
in the water).
II Adequate Not provided or inadequate
(e.g., stating that a lake was crowded).
III Provided, but limited Provided and adequate
a) Epidemiologic data were provided
that did not meet the criteria for Class I; or
b) the claim was made that ill persons had
no exposures in common besides water,
but no data were provided.
IV Provided, but limited Not provided or inadequate
----------------------------------------------------------------------------------------------------------
* The classification is based on the epidemiologic and water-quality data that were provided on
the outbreak-report form.
+ Adequate data were provided to implicate water as the source of the outbreak.
============================================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Waterborne-disease outbreaks associated with drinking water -- United States, 1993 (N=18) *
=========================================================================================================================================
No. of Type of
State Month Class + Etiologic agent cases system & Deficiency @ Source Setting
-----------------------------------------------------------------------------------------------------------------------------------
Arizona Jul -- ** Lead 1 Ind 4 Water-storage tank Private home
Arizona Aug -- Lead 1 Ind 4 Water-storage tank Private home
California Nov -- Lead 1 Ind 4 Water-storage tank Private home
Hawaii Oct I Fluoride 9 Com 4 Well Community
Indiana ? ++ I Nitrate 3 && Ind 2 Well Private homes
Minnesota Aug II Cryptosporidium 27 Ncom 5 @@ Lake Resort
parvum
Minnesota Nov I Campylobacter jejuni 32 Ncom 4 *** Well Resort
Missouri Nov I Salmonella serotype 625 Com 4 *** Well Community
Typhimurium
Mississippi Aug I Fluoride 34 Com 3 Well Community
Nevada +++ Dec &&& II C. parvum 103 @@@ Com 5 @@ Lake Community
New York Jun III Campylobacter jejuni 172 Com 5 Well Subdivision
Pennsylvania Jan III Giardia lamblia 20 Com 3 @@ Well Trailer park
Pennsylvania Mar I AGI **** 65 Ncom 3 Well Ski resort
Pennsylvania Mar I Copper 43 Com 4 @@ Reservoir Hotel
South Dakota Sep III G. lamblia 7 Com 2 Well Subdivision
South Dakota Sep I AGI 40 Ncom 2 Well Resort
Washington Apr III C. parvum 7 Ind 2 Well Private home
Wisconsin ++++ Mar I C. parvum 403,000 Com 3 &&&& Lake Community
-----------------------------------------------------------------------------------------------------------------------------------
* Refer to the Methods section for a description of the reporting variables.
+ Refer to Table 1 for information concerning the classification of outbreaks.
& Ncom=noncommunity; Com=community; Ind=individual; refer to the Methods section for definitions of the types of water systems.
@ Refer to the Methods section for the classification of water-system deficiencies.
** According to the classification scheme, single cases of illness resulting from chemical poisonings are not classified.
++ The month of onset is not known for this outbreak.
&& Three pregnant women had a total of six miscarriages.
@@ The water treatment included filtration.
*** The outbreak was associated with a contaminated water-storage tower.
+++ The outbreak occurred in Clark County, which includes Las Vegas.
&&& The outbreak period was estimated to extend from December 1993 through June 1994.
@@@ The outbreak involved mainly HIV-infected persons; estimates of the total number of infected persons in Clark County are not
available (refer to the text for details).
**** AGI=acute gastrointestinal illness of unknown etiology.
++++ The outbreak occurred in Milwaukee.
&&&& The water was inadequately filtered, but the treated water met all existing state and federal water-quality standards in effect at
that time.
=========================================================================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Waterborne-disease outbreaks associated with drinking water -- United States, 1994 (N=12) *
====================================================================================================================================
No. of Type of
State + Month Class & Etiologic agent cases system @ Deficiency ** Source Setting
----------------------------------------------------------------------------------------------------------------------------------
Idaho Jun III Shigella flexneri 33 Ind 2 Well Private homes
Indiana ? ++ -- && Nitrate 1 @@ Ind 2 Well Private home
Indiana Mar II AGI *** 118 Ncom 2 Well Restaurant
Maine Aug III AGI 72 Ncom 2 Well Camp
Minnesota Jun I Campylobacter jejuni 19 Ncom 2 Well Park
New Hampshire May III Giardia lamblia 18 Com 3 +++ Reservoir Community
New Hampshire May III G. lamblia 36 Com 3 +++ Lake Community
New York Jun III Shigella sonnei 230 Ncom 2 Well Camp
Northern Mariana Jun III Non-O1 Vibrio 11 Com 5 Wells &&& Bottled water
Islands (Saipan) cholerae
Pennsylvania Sep I AGI 200 Ncom 3 Well Resort
Tennessee Mar I G. lamblia 304 Com 4 Reservoir @@@ Correctional
facility
Washington Aug I Cryptosporidium 134 Com 2 Well Community
parvum
----------------------------------------------------------------------------------------------------------------------------------
* Refer to the Methods section for a description of the reporting variables.
+ This also includes one territory.
& Refer to Table 1 for information concerning the classification of outbreaks.
@ Ncom=noncommunity; Com=community; Ind=individual; refer to the Methods section for definitions of the types of water systems.
** Refer to the Methods section for the classification of water-system deficiencies.
++ The month of onset is not known for this outbreak.
&& According to the classification scheme, single cases of illness resulting from chemical poisonings are not classified.
@@ One pregnant woman had two miscarriages.
*** AGI=acute gastrointestinal illness of unknown etiology.
+++ Unfiltered surface water was used.
&&& Water was treated with reverse osmosis; refer to the text for details of the outbreak.
@@@ In addition, spring water, which was chlorinated but not filtered, was used for source water.
====================================================================================================================================
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Waterborne-disease outbreaks associated with drinking water, by etiologic
agent and type of water system -- United States, 1993-1994 (N=30) *
============================================================================================================
Type of water system +
---------------------------------------------------------------
Community Noncommunity Individual Total
------------------- ------------------ ------------------ -------------------
Etiologic agent Outbreaks Cases Outbreaks Cases Outbreaks Cases Outbreaks Cases
----------------------------------------------------------------------------------------------------------
Cryptosporidium
parvum 3 403,237 1 27 1 7 5 403,271
AGI & 0 0 5 495 0 0 5 495
Giardia lamblia 5 385 0 0 0 0 5 385
Campylobacter
jejuni 1 172 2 51 0 0 3 223
Lead 0 0 0 0 3 3 3 3
Fluoride 2 43 0 0 0 0 2 43
Nitrate 0 0 0 0 2 4 2 4
Salmonella
serotype
Typhimurium 1 625 0 0 0 0 1 625
Shigella sonnei 0 0 1 230 0 0 1 230
Copper 1 43 0 0 0 0 1 43
Shigella flexneri 0 0 0 0 1 33 1 33
Non-O1 Vibrio
cholerae 1 11 0 0 0 0 1 11
Total 14 404,516 9 803 7 47 30 405,366
(Percentage @) (46.7) (99.8) (30.0) (<1.0) (23.3) (<1.0) (100.0) (100.0)
----------------------------------------------------------------------------------------------------------
* Ordered by total number of outbreaks and secondarily by total number of cases.
+ Refer to the Methods section for definitions of the types of water systems.
& AGI=acute gastrointestinal illness of unknown etiology.
@ The percentage is based on 30 outbreaks or 405,366 cases.
============================================================================================================
Return to top. Table_5 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 5. Waterborne-disease outbreaks associated with drinking water, by type of
deficiency and type of water system -- United States, 1993-1994 (N=30)
=================================================================================================
Type of water system *
-------------------------------------------------
Community Noncommunity Individual Total
------------- ------------- ------------- -------------
Type of deficiency + No. (%) No. (%) No. (%) No. (%)
-----------------------------------------------------------------------------------------------
Untreated surface water 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0)
Untreated groundwater 2 ( 14.3) 5 ( 55.6) 4 ( 57.1) 11 ( 36.7)
Treatment 5 ( 35.7) 2 ( 22.2) 0 ( 0.0) 7 ( 23.3)
Distribution system 4 ( 28.6) 1 ( 11.1) 3 ( 42.9) 8 ( 26.7)
Unknown 3 ( 21.4) 1 ( 11.1) 0 ( 0.0) 4 ( 13.3)
Total 14 (100.0) 9 (100.0) 7 (100.0) 30 (100.0)
-----------------------------------------------------------------------------------------------
* Refer to the Methods section for definitions of the types of water systems.
+ Refer to the Methods section for the classification of water-system deficiencies.
=================================================================================================
Return to top. Figure_1 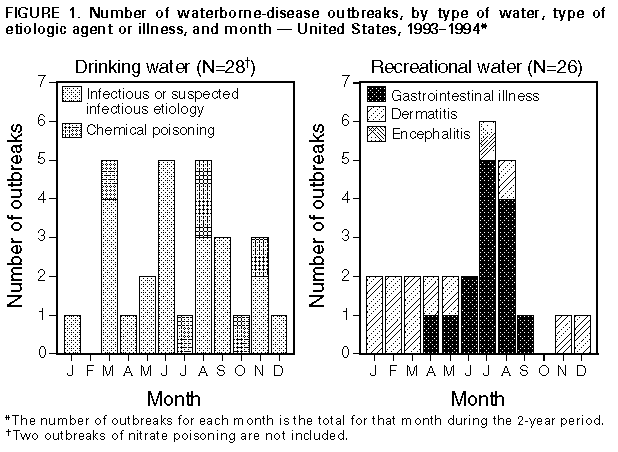 Return to top. Figure_2 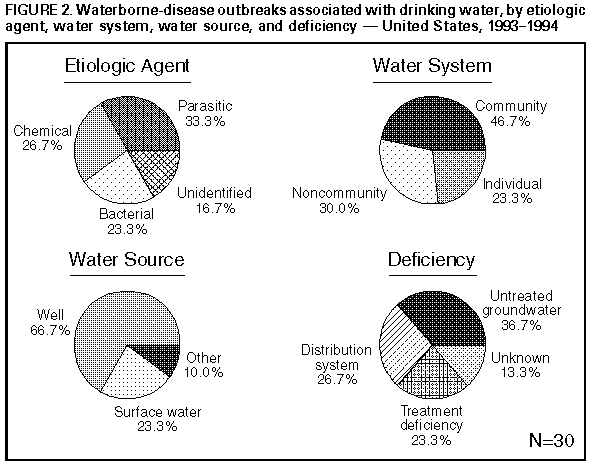 Return to top. Table_6 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 6. Waterborne-disease outbreaks of gastroenteritis and meningoencephalitis associated with recreational water -- United
States, 1993-1994 (N=15)
=========================================================================================================================
No. of
State Year Month Class * Illness Etiologic agent cases Source Setting
-----------------------------------------------------------------------------------------------------------------------
Indiana 1994 Jun I Gastroenteritis Giardia lamblia 80 Pool Community
Maryland 1993 Jul II Gastroenteritis G. lamblia 12 Lake Park
Minnesota 1994 May II Gastroenteritis Shigella flexneri 35 Lake Park
Missouri 1994 Jul I Gastroenteritis Cryptosporidium parvum 101 Pool Motel
New Jersey 1993 Sep IV Gastroenteritis G. lamblia 43 Lake Swim club
New Jersey 1994 Jun II Gastroenteritis Shigella sonnei 242 Lake Park
New Jersey 1994 Jul I Gastroenteritis C. parvum 418 Lake Park
New York 1994 Jul II Gastroenteritis Escherichia coli O157:H7 166 Lake Camp
Ohio 1993 Jul III Gastroenteritis S. sonnei 160 Lake Park
Texas 1994 Jul -- + Meningoencephalitis Naegleria fowleri 1 Pond/river Rural area
Washington 1993 Aug III Gastroenteritis G. lamblia 6 River River
Wisconsin 1993 Apr II Gastroenteritis C. parvum 51 Pool Motel
Wisconsin 1993 Aug II Gastroenteritis C. parvum 5 Pool Community
Wisconsin 1993 Aug II Gastroenteritis C. parvum 54 Pool Community
Wisconsin 1993 Aug II Gastroenteritis C. parvum 64 Pool Motel
-----------------------------------------------------------------------------------------------------------------------
* Refer to Table 1 for information concerning the classification of outbreaks.
+ According to the classification scheme, single cases of primary amebic meningoencephalitis are not classified.
=========================================================================================================================
Return to top. Table_7 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 7. Waterborne-disease outbreaks of dermatitis associated with recreational water -- United States, 1993-1994 (N=11)
======================================================================================================
No. of
State Year Month Class * Etiologic agent cases Source Setting
----------------------------------------------------------------------------------------------------
Alaska 1993 Aug -- + Pseudomonas 4 @ Hot tub Bed & breakfast
aeruginosa &
Alaska 1993 Dec -- P. aeruginosa 2 Pool/whirlpool Resort
Colorado 1994 Feb -- P. aeruginosa 10 Hot tub Ski resort
Minnesota 1993 Jan -- P. aeruginosa & 14 Hot tub Hotel
Minnesota 1993 Mar -- P. aeruginosa & 6 Hot tub Hotel
Minnesota 1993 Apr -- P. aeruginosa & 15 Pool School
Minnesota 1993 May -- P. aeruginosa 53 @ Pool School
Minnesota 1994 Jan II Chemical ** 9 Pool Hotel
Minnesota 1994 Feb -- P. aeruginosa 30 Pool Hotel
South Dakota 1994 Mar -- P. aeruginosa 113 ++ Pool/hot tub Lodge
Utah 1994 Nov IV Chemical ** 20 Pool Hospital
----------------------------------------------------------------------------------------------------
* Refer to Table 1 for information concerning the classification of outbreaks.
+ According to the classification scheme, outbreaks of pseudomonas dermatitis are not classified.
& The clinical syndrome was consistent with that caused by P. aeruginosa.
@ This includes patients with otitis externa.
** The clinical syndrome was suggestive of a chemical etiology.
++ This includes patients with otitis externa and pharyngitis.
======================================================================================================
Return to top. Figure_3 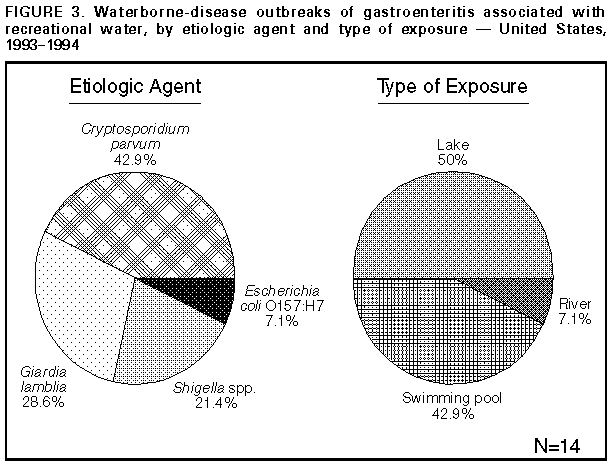 Return to top. Table_8 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 8. Waterborne-disease outbreaks associated with drinking water that were not
included in the previous surveillance summary -- United States, 1992 (N=8) *
===========================================================================================================
Etiologic No. of Type of
State Month Class + agent cases system & Deficiency @ Source Setting
---------------------------------------------------------------------------------------------------------
Minnesota May I AGI ** 70 NCom 5 Well Hotel
Missouri Jan III Shigella 5 Ind 4 Cistern Private home
sonnei
Missouri Apr III Hepatitis 46 NCom 2 Well School/
A virus church
Missouri Jul I S. sonnei 11 NCom 4 Well Campground
Pennsylvania May I AGI 50 NCom 3 Well Camp
Wisconsin Jan I Copper 24 Com 4 Well Subdivision
Wisconsin Sep I Copper 8 Com 4 Lake Building
Wisconsin Dec III Copper 6 Com 5 Well House
---------------------------------------------------------------------------------------------------------
* Refer to the Methods section for a description of the reporting variables.
+ Refer to Table 1 for information concerning the classification of outbreaks.
& NCom=noncommunity; Com=community; Ind=individual; refer to the Methods section
for definitions of the types of water systems.
@ Refer to the Methods section for the classification of water-system deficiencies.
** AGI=acute gastrointestinal illness of unknown etiology.
===========================================================================================================
Return to top. Table_9 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 9. Waterborne-disease outbreaks associated with recreational water that were
not included in the previous surveillance summary -- United States, 1992 (N=4) *
=========================================================================================================
No. of
State Month Class + Illness Etiologic agent cases Source Setting
-------------------------------------------------------------------------------------------------------
California Aug I Gastroenteritis AGI & 100 Pool Water park
New Jersey Jun I Gastroenteritis Shigella sonnei 54 Lake Campground
New Jersey Jul III Dermatitis Schistosoma sp. @ 40 Lake Lake
Virginia Jul III Gastroenteritis S. sonnei 9 Lake Camp
-------------------------------------------------------------------------------------------------------
* Refer to the Methods section for a description of the reporting variables.
+ Refer to Table 1 for information concerning the classification of outbreaks.
& AGI=acute gastrointestinal illness of unknown etiology.
@ The clinical syndrome was consistent with that caused by a Schistosoma sp.
=========================================================================================================
Return to top. Figure_4 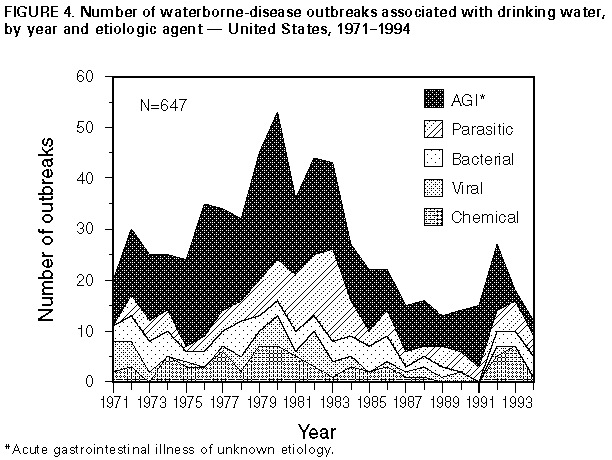 Return to top. Figure_5 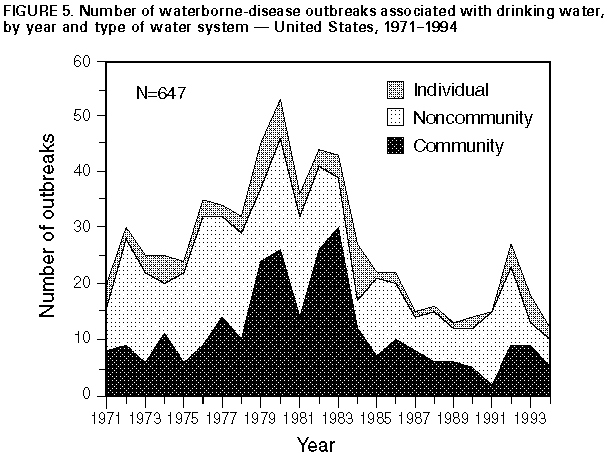 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|