 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Testing for Antibodies to Human Immunodeficiency Virus Type 2 in the United StatesThis report was prepared by the following: Thomas R. O'Brien, M.D., M.P.H. * J. Richard George, Ph.D. * Jay S. Epstein, M.D. ** Scott D. Holmberg, M.D., M.P.H. * Gerald Schochetman, Ph.D. *
** Food and Drug Administration Summary The Food and Drug Administration (FDA) has recommended that all donated blood be screened for antibodies to human immunodeficiency virus type 2 (HIV-2) beginning no later than June 1, 1992. This article provides CDC recommendations for the diagnosis of HIV-1 and HIV-2 infections in persons being tested in settings other than blood centers and CDC/FDA guidelines for serologic testing with combination HIV-1/HIV-2 screening enzyme immunoassays (EIAs). Epidemiologic data indicate that the prevalence of HIV-2 infections in persons in the United States is extremely low. Therefore, CDC does not recommend routine testing for HIV-2 in settings other than blood centers. However, when HIV testing is indicated, tests for antibodies to both HIV-1 and HIV-2 should be obtained if epidemiologic risk factors for HIV-2 infection are present, if clinical evidence exists for HIV disease in the absence of a positive test for antibodies to HIV-1, or if HIV-1 Western blot results exhibit the unusual indeterminate pattern of gag plus pol bands in the absence of env bands. The following procedures are recommended if testing for both HIV-1 and HIV-2 is performed by means of a combination HIV-1/HIV-2 EIA. A repeatedly reactive specimen by HIV-1/HIV-2 EIA should be tested by HIV-1 Western blot (or another licensed HIV-2 supplemental test). A positive result by HIV-1 Western blot confirms the presence of antibodies to HIV, and testing for HIV-2 is recommended only if HIV-2 risk factors are present. If the HIV-1 Western blot result is negative or indeterminate, an HIV-2 EIA should be performed. If the HIV-2 EIA is positive, an HIV-2 supplemental test should be performed. INTRODUCTION Efforts to prevent transmission of human immunodeficiency virus type 1 (HIV-1), particularly through the blood supply, led to the rapid development in 1985 of diagnostic tests for HIV-1 antibodies. In 1986, a second virus causing the acquired immunodeficiency syndrome (AIDS), human immunodeficiency virus type 2 (HIV-2), was discovered and found to be relatively common in parts of West Africa (1-3). Because HIV-2 infections are not always detected by HIV-1 antibody tests (4), antibody tests for HIV-2 have been developed. On April 25, 1990, the Food and Drug Administration (FDA) licensed an enzyme immunoassay (EIA) test kit for detection of antibodies to HIV-2 in human serum or plasma. The test, Genetic Systems HIV-2 EIA, manufactured and distributed by Genetic Systems Corp., Redmond, WA, is based on a disrupted whole-virus antigen obtained by purification of HIV-2 grown in cell culture. Licensure of the HIV-2 EIA raised the possibility of routine donor screening for HIV-2. However, after public discussion at the FDA Blood Products Advisory Committee meeting in March 1990, FDA decided not to recommend routine anti-HIV-2 screening of blood donated for transfusion. This decision was based on the collective evidence that HIV-2 infection in the United States was extremely rare (5). There was also reluctance to increase the complexity of testing performed by blood centers by introducing an additional test, of limited usefulness, to the battery of tests already being performed. However, voluntary screening for HIV-2 antibodies by blood banks was considered to be an acceptable practice. On September 25, 1991, FDA licensed the Genetic Systems HIV-1/HIV-2 EIA and on February 14, 1992, licensed the HIVAB HIV-1/HIV-2 (rDNA) EIA (Abbott Laboratories, North Chicago, IL). These tests permit simultaneous testing for both HIV-1 and HIV-2 without increasing the number of screening tests performed by blood banks. In accordance with FDA recommendations effective June 1, 1992, blood centers have begun testing all donated whole blood, blood components, and source plasma for antibodies to HIV-2 (FDA: Revised recommendations for the prevention of human immunodeficiency virus {HIV} transmission by blood and blood products {memorandum}. Bethesda, MD: Center for Biologics Evaluation and Research, FDA, April 23, 1992). Blood centers can accomplish this either by the use of a single combination test for HIV-1/HIV-2 or by the use of two independent tests, one for HIV-1 and one for HIV-2. Screening donated blood and plasma for HIV-2 infection raises issues concerning appropriate strategies for testing for both viruses, HIV-2 testing in other settings, and notification of HIV-1 and HIV-2 test results. This article provides CDC recommendations for the diagnosis of HIV-1 and HIV-2 infections in persons being tested in settings other than blood centers and CDC/FDA guidelines for serologic testing with combination HIV-1/HIV-2 screening EIAs. EPIDEMIOLOGIC STUDIES Although HIV-2 appears to have spread in West Africa primarily via heterosexual transmission (3), HIV-2 infection has been reported in Europe in homosexual men (6), injecting drug users (IDUs) (7), transfusion recipients (8,9), and men with hemophilia (10). HIV-2 is endemic in parts of West Africa (3, 11, 12) and has also been reported in other parts of Africa (3) Table_1. Apparently as a result of links with former colonies in West Africa, Portugal and France have reported the highest number of cases of HIV-2 infection in Europe (13). As of late 1989, 12.6% of AIDS cases in Portugal were caused by HIV-2 (14). Although most of these cases were in persons originally from Africa, HIV-2 is also present among persons in Portugal with no known contacts with Africa. HIV-2 infection has also been reported in India (15). In the Western hemisphere, rare cases of HIV-2 infection have been reported from Brazil(16, 17), Canada (18), and the United States (5). Within the United States, CDC and others conduct surveillance for HIV-2, including serologic surveillance of blood donors and populations at increased risk of HIV-1 infection. Since 1987, 32 persons with HIV-2 infection have been reported in the United States. Fifteen of these 32 were identified by serologic surveillance, and 17 were identified by case reports. Twenty-eight were residing in the northeastern United States (5), a frequent destination for West African immigrants and the area that has been most intensely surveyed using HIV-2-specific tests. No cases of HIV-2 infection have been reported among persons known to be IDUs or men reporting homosexual contact (5). More than 2,700 serum specimens that were reactive by HIV-1 EIA and indeterminate by HIV-1 Western blot have been tested for HIV-2 by either the New York City Health Department or the Maryland Department of Health and Mental Hygiene (5, 19). HIV-2 infection was detected in specimens from 11 persons. The Massachusetts Department of Public Health (5) identified two HIV-2-positive specimens among blood samples from 14,779 childbearing women. Positive HIV-2 specimens were detected among sera from two of 19,504 clients of sexually transmitted disease clinics, but in none of the specimens from 6,547 IDUs at drug-treatment centers (20). In other studies of populations at increased risk for HIV-1 infection, no cases of HIV-2 infection have been reported (5). Of 15 U.S. residents found to be positive for HIV-2 infection through serologic surveillance, demographic information was available for seven; six were West Africans and one was the U.S.-born wife of an HIV-2 infected West African (5). Most of the 17 persons identified by case reports were West Africans residing in the United States, but one was a U.S. resident of European origin and two were native-born U.S. citizens (5). All three non-West Africans had traveled to West Africa. One native-born U.S. citizen was diagnosed as HIV-2 infected after volunteering to donate blood in 1986. However, serologic surveillance of more than 26,000,000 blood donations collected between 1987 and 1991 has not revealed another instance of an HIV-2 infected U.S. blood donor (21-25). DIAGNOSIS OF HIV-2 INFECTION Although considerable serologic cross-reaction occurs between HIV-1 and HIV-2, HIV-2 infection may not be diagnosed when screening is done exclusively with HIV-1 tests. From 60% to 91% of HIV-2-infected persons will test repeatedly reactive by HIV-1 whole-virus lysate EIA (4). According to data provided to FDA by the test manufacturers, HIV-2 antibodies are detected with >99% sensitivity by FDA-licensed HIV-1/HIV-2 EIAs and the FDA-licensed HIV-2 EIA. However, analogous to the diagnosis of HIV-1 infection, diagnosis of HIV-2 infection requires more specific supplemental tests, such as an HIV-2 Western blot. Although no licensed supplemental tests exist for HIV-2 infection, research tests are available. Several European and U.S. biotechnology companies manufacture HIV-2 Western blot tests. Studies of these tests have been performed by manufacturers of HIV test kits, by state and local public health laboratories, and by CDC (26,27). The diversity of protein bands, especially glycoprotein bands, is greater on the HIV-2 Western blot tests than on HIV-1 Western blot tests (28). This variation occurs because the various HIV-2 Western blot tests use different strains of HIV-2 and because of the different methods by which HIV-2 antigens are prepared before separation by electrophoresis (28). No one test appears to have a distinct advantage. Therefore, although public health laboratories would benefit from a standard HIV-2 Western blot test format to which a single set of interpretive criteria could be applied, a single standard currently cannot be applied to all tests. CDC recommends that each test be interpreted by the criteria suggested by the kit manufacturer. Other investigational tests for antibodies to HIV-2, such as immunofluorescence (29), EIAs based on HIV-1 and HIV-2 synthetic peptides (30), and radioimmune precipitation (31), can be useful in distinguishing a true positive HIV-2 EIA screening test result. Gene amplification by polymerase chain reaction (17) and viral culture (32) can also be used to determine the virus type. RECOMMENDATIONS FOR HIV-2 TESTING IN THE UNITED STATES Indications for Testing for HIV-2 Infection Because epidemiologic data indicate that the prevalence of HIV-2 in the United States is extremely low, CDC does not recommend routine testing for HIV-2 at U.S. HIV counseling and test sites or in settings other than blood centers. However, when HIV testing is to be performed, tests for antibodies to both HIV-1 and HIV-2 should be obtained if demographic or behavioral information suggests that HIV-2 infection might be present. Persons at risk for HIV-2 infection include:
evidence for or suspicion of HIV disease (such as an AIDS-associated opportunistic infection)in the absence of a positive test for antibodies to HIV-1 and in cases in which the HIV-1 Western blot exhibits the unusual indeterminate pattern of gag (p55, p24, or p17) plus pol (p66, p51, or p32) bands in the absence of env (gp160, gp120, Or gp41) bands. Other Considerations The potential risk of HIV-2 infection in some populations (such as those described above) may justify routine HIV-2 testing for all persons for whom HIV-1 testing is warranted. The decision to implement routine HIV-2 testing requires consideration of the number of HIV-2-infected persons who would remain undiagnosed without routine HIV-2 testing compared with the problems and costs associated with its implementation. Because implementation of routine HIV-2 testing would increase the number of tests performed on some specimens and because confirmatory testing for HIV-2 would be limited to laboratories that perform nonlicensed HIV-2 supplemental tests, the maximum "turnaround" time required to complete HIV testing would increase for some specimens. At HIV counseling and test sites, clients might require an additional appointment after the routine post-test counseling session to receive HIV-2 test results and HIV-2 post-test counseling. Another factor to consider when routine HIV-2 testing is being contemplated is the predictive value of HIV-2 antibody screening tests in most U.S. populations. Given the extremely low prevalence of HIV-2 in the United States, very few persons who test positive by HIV-2 antibody screening tests will actually be HIV-2 infected In addition, HIV-2 testing may identify persons with indeterminate HIV-2 test results that must be explained to the patient and appropriate follow-up initiated. Finally, implementation of routine HIV-2 testing would increase HIV testing costs, as HIV-1/HIV-2 combination EIAs are more expensive than HIV-1 EIAs, and testing with HIV-2 EIAs and supplemental tests would be required for some specimens. GUIDELINES FOR SEROLOGIC TESTING WITH COMBINATION HIV-1/HIV-2 SCREENING EIAs Laboratories that use a licensed combination HIV-1/HIV-2 screening test should follow the testing algorithm recommended by CDC and FDA Figure_1. If a combination test for HIV-1/HIV-2 is performed and is repeatedly reactive, additional, more specific testing is necessary to confirm the presence of antibodies either to HIV-1 or HIV-2 as follows:
MEDICAL COUNSELING Infection with either HIV-1 or HIV-2 can cause immunosuppression and the development of AIDS (34,35). Although the period between infection and disease may be longer for persons with HIV-2 than for those with HIV-1 (36,37), the modes of transmission and, therefore, preventive counseling are the same for persons with either virus. Furthermore, because data are limited regarding the effectiveness of antiviral therapy for HIV-2 infection, persons with a confirmed antibody test for HIV-2 should be managed similarly to persons with a confirmed antibody test for HIV-1. Additional testing to define the virus type is of epidemiologic importance and should be considered for persons with epidemiologic risk factors for infection with HIV-2. Based on the epidemiology and prevalence of HIV-2 in the United States, CDC/FDA makes the following recommendations for notification of persons with repeatedly reactive combination screening tests for HIV-1/HIV-2.
References
Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. African countries with a high prevalence of HIV-2 infection (11, 12)
=============================================================================
West African nations Mauritania *
Benin Niger
Burkina Faso Nigeria *
Cape Verde * Sao Tome
Cote d'Ivoire * Senegal
Gambia * Sierra Leone *
Ghana Togo
Guinea
Guinea-Bissau * Other African nations
Liberia Angola *
Mali * Mozambique *
-----------------------------------------------------------------------------
* Prevalence of HIV-2 reported to exceed 1% in the general population.
Return to top. Figure_1 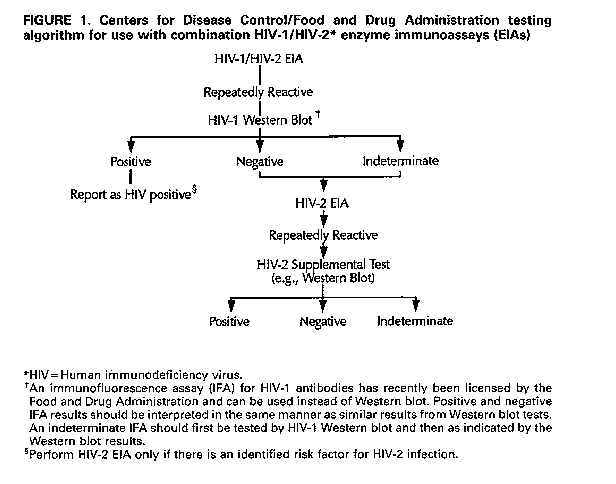 Return to top. Disclaimer All MMWR HTML documents published before January 1993 are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 08/05/98 |
|||||||||
This page last reviewed 5/2/01
|