 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Management of Persons Exposed to Multidrug-Resistant TuberculosisThis report was prepared by the following CDC staff: Margarita E. Villarino, M.D., M.P.H. Samuel W. Dooley, Jr., M.D. Lawrence J. Geiter, M.P.H. Kenneth G. Castro, M.D. Dixie E. Snider, Jr., M.D., M.P.H. Summary Recent outbreaks of multidrug-resistant tuberculosis (MDR-TB) have posed challenges for the management of exposed persons. This report offers suggestions for evaluating and managing persons (i.e., contacts) who have been exposed to patients with infectious MDR-TB (TB due to strains of Mycobacterium tuberculosis resistant to both isoniazid {INH} and rifampin {RIF}), provides background information on alternative preventive therapy regimens with drugs other than INH or RIF, and presents considerations relevant to making a decision to offer one of these alternative regimens. INTRODUCTION During 1990 and 1991, four outbreaks of multidrug-resistant tuber- culosis (MDR-TB), involving nearly 200 cases, were investigated by CDC and local health departments in Florida and New York City (1,2). These outbreaks were characterized by transmission of Mycobacterium tubercu- losis to patients and to health-care workers (HCWs) in hospitals and outpatient clinics; most of the exposed persons who developed active MDR-TB were known to be infected with human immunodeficiency virus (HIV). Among patients with active MDR-TB, the case-fatality rate was extraordinarily high -- 72% to 89%. Of eight HCWs from these hospitals who developed active MDR-TB, four with known HIV infection died. Since August 1991, CDC and state and local health departments in New York have also investigated MDR-TB outbreaks involving two additional hospitals and the state correctional system. In these outbreaks, several inmates and one prison guard have died of MDR-TB (CDC: unpublished data). Approximately 50 HCWs in one of these hospitals had tuberculin skin-test conversions after exposure to hospitalized patients with MDR-TB. Virtually all M. tuberculosis isolates from the MDR-TB outbreak cases were resistant to both isoniazid (INH) and rifampin (RIF); some were resistant to other drugs, including ethambutol (EMB), strepto- mycin (SM), ethionamide, kanamycin (KM), and rifabutin. Data for susceptibility to pyrazinamide (PZA) are incomplete, but for those isolates in which PZA testing has been completed, most were susceptible to this drug. PATHOGENESIS AND PREVENTION OF TUBERCULOSIS Persons with recently acquired M. tuberculosis infection are at relatively high risk of developing active TB; in general, 5%-10% of persons develop active disease within 2 years of the primary infection (3-5). Coinfection with HIV increases this risk considerably; seven (41%) of 18 HIV-infected patients identified in a nosocomial TB outbreak investigation in Italy developed active disease within 60 days of their exposure to M. tuberculosis, and 11 (38%) of 29 persons in a residential facility for HIV-infected persons in San Francisco developed active disease within 4 months of exposure (6-8). To reduce the risk of active TB in persons newly infected with M. tuberculosis, the American Thoracic Society/CDC and the Advisory Council for the Elimination of Tuberculosis recommend INH preventive therapy (9-10). For HIV-infected persons, the higher disease attack rate and the shorter incubation period associated with newly acquired tuberculous infection and the high mortality rate associated with TB disease reinforce the rationale for the use of preventive therapy. In HIV-infected persons who become newly infected with M. tuberculosis, the use of drug therapy might be considered treatment of incubating or subclinical disease. When the infecting strain of M. tuberculosis is susceptible to INH and patients adhere to the drug regimen, INH is highly effective for preventing active TB. In a wide variety of controlled studies, persons who were prescribed 12 months of INH preventive therapy had a 30%-93% reduction in the rate of active disease; the variation in effec- tiveness was almost entirely due to variation in patient adherence to the prescribed regimen (11,12). In a more recent study, nursing-home residents in Arkansas who received 12 months of INH preventive therapy after a documented tuberculin skin-test conversion had a 98% reduction in the rate of active disease, compared with those who had a skin-test conversion but did not take preventive treatment (3). Most studies of preventive therapy have been conducted among immunocompetent subjects. However, early results of a recent study in Zambia indicated the efficacy of 6 months of INH preventive therapy in tuberculin-positive persons coinfected with HIV. This study showed a substantial reduction (87%) in the rate of active disease in the treated group when compared with a control group that received placebo (13). RIF is recommended as an alternative to INH when the infecting strain of M. tuberculosis is resistant to INH but susceptible to RIF (9). When the infecting strain is multidrug resistant (i.e., resistant to both INH and RIF), treatment options are problematic because no studies have demonstrated the effectiveness of preventive therapy for persons infected with such strains of M. tuberculosis. IDENTIFICATION AND EVALUATION OF PERSONS EXPOSED TO TUBERCULOSIS Several factors should be considered in the management of persons exposed to TB (i.e., contacts): a) the likelihood that the contact is newly infected with M. tuberculosis; b) the likelihood that the infecting strain of M. tuberculosis is multidrug resistant; and c) the estimated likelihood that the contact, if infected, will develop active TB. Estimating the Likelihood of New Infection with M. tuberculosis and Preventive Therapy Decision-making Because contacts of persons with infectious TB are at high risk for tuberculous infection, contacts should be rapidly identified and evaluated when a case of infectious TB (i.e., TB involving the respiratory tract or oral cavity) is identified (Figure_1) (14). Contacts who are not known or likely to be immunosuppressed and who have a documented prior positive tuberculin skin test (Mantoux test with 5 tuberculin units of purified protein derivative {PPD}) are probably not newly infected. These persons need no further evaluation for the current TB exposure unless they have symptoms suggestive of active TB; however, they should be evaluated for preventive therapy on the basis of the prior positive tuberculin skin-test result (10). Contacts who are not known or likely to be immunosuppressed and who have no history of a positive tuberculin skin-test reaction should receive a tuberculin skin test and, if indicated, chest radiograph and sputum examination. Those who have tuberculin skin-test reactions with greater than or equal to 5 mm induration should be considered probably newly infected with M. tuberculosis and should be evaluated for preventive therapy, after active TB is excluded (Figure_1) (15). Members of the immediate family, close social contacts, or others (e.g., hospital roommates, some health-care providers) who shared the same indoor environment with an infectious TB patient for substantial periods are considered high-risk contacts. High-risk contacts who have negative tuberculin skin-test reactions (<5 mm induration) and who are not anergic should receive follow-up skin tests 12 weeks after their exposure to TB has ended. These initially tuberculin-negative, high- risk contacts should be considered possibly newly infected. They should receive a chest radiograph and be considered for preventive therapy until follow-up testing is complete if a) there is evidence of TB contagion among other contacts with comparable exposure (i.e., tuberculin positivity or active TB), or b) the contact is a child, HIV infected, or immunosuppressed for other reasons. Preventive therapy can be discontinued if follow-up tuberculin skin-test results are negative. Contacts who are known or likely to be HIV infected, or markedly immunosuppressed for other reasons, should be evaluated for anergy at the time of tuberculin skin testing (Figure_1) (16). Immunosup- pressed persons who have a prior positive tuberculin skin test may be at risk for reinfection with M. tuberculosis; these persons should also be evaluated with a tuberculin skin test and a test for anergy. Those who are not anergic can be further evaluated in the same way as contacts who are not known or likely to be immunosuppressed. Those who are anergic should be considered probably newly infected if they are high-risk contacts or if there is evidence of TB contagion among other contacts with comparable exposure. (The latter suggests that the source case was an efficient transmitter.) These persons should be evaluated for preventive therapy, after active TB is excluded. In all situations, regardless of tuberculin skin-test results, a diagnostic evaluation should be performed if symptoms suggestive of active TB are present. Estimating the Likelihood of Infection with Multidrug-Resistant M. tuberculosis Among Contacts Thought to Be Newly Infected Contacts who are considered likely to be newly infected and who have had exposure to patients with infectious MDR-TB should be evaluated to assess the likelihood of infection with a multidrug- resistant strain of M. tuberculosis. Several factors should be considered in this assessment: a) the infectiousness of the possible source MDR-TB case, b) the closeness and intensity of the MDR-TB exposure, and c) the contact's likelihood of exposure to persons with drug-susceptible TB (Table_1). Infectiousness of the source case. TB patients who cough and have acid-fast bacillus (AFB) smear-positive sputum are substantially more infectious than those who do not cough and have AFB smear-negative sputum. Evidence of tuberculin skin-test conversions among contacts of the TB case is another measure of infectivity. Closeness and intensity of the MDR-TB exposure. Any person who shared the air space with an MDR-TB patient for a relatively prolonged time (e.g., household member, hospital roommate) is at higher risk for infection than those with a brief exposure to an MDR-TB patient, such as a one-time hospital visitor. Exposure of any length in a small, enclosed, poorly ventilated area is more likely to result in trans- mission than exposure in a large, well-ventilated space. Exposure during cough-inducing procedures (e.g., bronchoscopy, endotracheal intubation, sputum induction, administration of aerosol therapy), which may greatly enhance TB transmission, is also more likely to result in infection. Contact history. Persons exposed to several sources of M. tubercu- losis, including infectious TB patients with drug-susceptible M. tuberculosis, are less likely to have been infected with a multidrug- resistant strain than are those whose only known exposure to TB was to an infectious MDR-TB case. Estimating the Likelihood that Infected Persons Will Develop Active Tuberculosis The most potent factor that increases the probability that a person infected with M. tuberculosis will develop active TB is immunodeficiency, such as that caused by coinfection with HIV (Table_2) (17,18); thus, health-care providers should routinely offer counseling and voluntary HIV antibody testing to all patients known or likely to be infected with M. tuberculosis. Other immunocom- promising conditions, including treatment with immunosuppressive medications, renal failure, and diabetes mellitus, also increase the risk for progression to active disease, but to a considerably lesser extent than HIV infection (9,18). Recentness of infection also contributes to the risk of developing active TB. In immunocompetent persons, the risk of developing active TB is highest within the first 2 years after infection, after which the risk declines markedly (18). This is probably different for HIV- infected persons, who have a progressive decline in cell-mediated immunity and may remain at high risk for an indefinite period or may even have an increasing risk as the immunosuppression progresses. Finally, the age of the contact needs to be considered. Children ages less than or equal to 5 years and persons greater than or equal to 60 years both have high TB disease attack rates and shorter incubation periods (3,19). PREVENTIVE THERAPY CONSIDERATIONS FOR PERSONS LIKELY TO BE INFECTED WITH A MULTIDRUG-RESISTANT STRAIN OF MYCOBACTERIUM TUBERCULOSIS Before any preventive therapy regimen is initiated, the diagnosis of clinically active TB must be excluded (14,20). Patients on preventive therapy should be monitored carefully for adverse reactions to the medications, evidence of active TB, and adherence to therapy. Patients on preventive therapy should be thoroughly educated about symptoms of TB and the need for immediate medical evaluation if symptoms do occur. As much as possible, alternative multidrug preventive therapy regimens should be selected, administered, and evaluated in a consistent and systematic way. All patients receiving one of these regimens should be on directly observed therapy. Clinicians who are not familiar with the management of patients with MDR-TB or those infected with multidrug-resistant organisms should seek expert consultation. Considerations in Evaluating M. tuberculosis Isolates for Drug Susceptibility Drug susceptibility test results of the M. tuberculosis isolate of the presumed source case should be considered in the selection of the drugs to be included in the preventive therapy regimen. The proportion method is commonly used for determining drug susceptibility of M. tuberculosis isolates (21). The results of this method of testing are reported to the clinician as the percentage of the total bacterial population resistant to a given drug, which is defined by the amount of growth on a drug-containing medium as compared with growth on a drug-free control medium. When greater than or equal to 1% of the bacillary population becomes resistant to the critical concentration of a drug, the M. tuberculosis isolate is considered resistant to that drug. The critical concentration of a drug is the concentration that inhibits the growth of most cells in wild strains of M. tuberculosis. One critical concentration is used for the susceptibility testing of most drugs; INH susceptibility testing is usually done by using two different drug concentrations (0.2 ug/mL and 1.0 ug/mL). Drug susceptibility testing of M. tuberculosis isolates is also possible through the use of radiometric techniques such as the BACTEC(R) system, Results of this method identify whether M. tubercu- losis isolates are susceptible or resistant to the drugs tested. This method can reduce the time of identifying drug-resistant microor- ganisms from the 7 weeks needed for testing in solid media (proportion method) to 3 weeks. However, only five of the antituberculosis drugs (INH, RIF, PZA, EMB, and SM) are commercially available at this time for use in the BACTEC(R) system. Additional antibiotics may be tested with BACTEC(R) but require that dilutions be prepared by the laboratory. General Approach to Selecting Drug Regimens for Preventive Therapy Candidates Newly infected contacts who are thought to have a low to low- intermediate likelihood of infection with multidrug-resistant M. tuberculosis (Table_1) should be managed according to standard recommendations for infected contacts of non-MDR-TB patients (Figure_2) (10). Infected contacts who are thought to have an intermediate to high likelihood of being infected with a multidrug- resistant strain of M. tuberculosis need to be further categorized according to their risk of developing active TB. Those at high risk for disease include persons who are HIV infected, persons with risk factors for HIV infection who have unknown HIV status, and persons with other conditions known to cause severe immunosuppression. Preventive therapy regimens with at least two antituberculosis drugs should be strongly considered for persons likely to be infected with multidrug-resistant M. tuberculosis who have a high risk of developing active disease (Figure_2). This suggestion is based on consider- ations of the extraordinarily high likelihood of progression to clinically active TB, especially in persons with advanced HIV disease; the severe consequences of clinically active MDR-TB in persons with HIV infection; and the possibility that MDR-TB may be more amenable to cure if treated when the bacterial burden is low rather than when the disease becomes clinically apparent and the bacterial burden is substantially higher. No clinical data exist on the risks and benefits of regimens that do not include INH or RIF; however, preventive therapy with more than one drug is recommended since efficacy of preventive monotherapy with alternative drugs has not been demon- strated. For contacts who are not HIV infected, who do not have risk factors for HIV infection with unknown HIV status, who do not have another condition that substantially increases the likelihood of progression to active TB, and who are believed to be infected with a strain of M. tuberculosis that is 100% resistant (by the proportion method of susceptibility testing) to INH and RIF, other considerations apply (Figure_2). For these persons, two options are available to the clinician: a) administer no preventive therapy and provide careful clinical follow-up for the appearance of signs and symptoms of TB, or b) consider multidrug preventive therapy with drugs other than INH or RIF. If the infecting strain of M. tuberculosis from the source patient is less than 100% resistant to INH, INH should be used for preventive therapy for contacts; alternatively, if the infecting strain is less than 100% resistant to RIF, RIF should be used (9). Because the efficacy of preventive therapy with drugs other than INH and RIF is unknown, persons placed on alternative multidrug preventive therapy regimens should receive periodic medical and radiographic evaluation for the first 2 years after tuberculous infection (e.g., every 3 months for persons who are HIV positive or who for some other reason have a substantially higher risk of developing active disease; every 6 months for persons who are HIV negative or are not at increased risk). Sputum examination should be done if TB symptoms are present. Duration of Preventive Therapy The suggested duration of alternative multidrug preventive therapy ranges from 6 to 12 months of continuous treatment. Twelve months of therapy are recommended for persons who have HIV infection or other immunosuppressive conditions that markedly increase the risk of developing active disease. Others should receive at least 6 months of continuous therapy. Potential Alternative Regimens of Preventive Therapy The alternative regimens of preventive therapy suggested in this document do not represent approval from the Food and Drug Adminis- tration (FDA) or approved labeling for the particular products or indications in question. In the United States, the only drug approved by the FDA for TB preventive therapy is INH. Alternative drugs should be used for preventive therapy only when susceptibility to the drugs has been demonstrated by testing the M. tuberculosis isolate of the presumed source case. One potential alternative preventive therapy regimen is a combin- ation of PZA and EMB at the recommended dose for the therapy of TB (PZA, daily oral dose 25-30 mg/kg; EMB, daily oral dose 15-25 mg/kg) (9). Preventive therapy studies in a mouse model suggest that PZA may enhance the efficacy of a bactericidal drug, since a PZA-RIF combin- ation was more effective than RIF alone (22). Both PZA and EMB have proved to be effective and well tolerated for the treatment of TB. EMB at the 15-mg/kg dose is considered bacteriostatic, and in vitro studies show that EMB may be bactericidal at the 25-mg/kg dose (23). If EMB is used at the higher dosage as part of a preventive therapy regimen, the potential for higher rates of toxicity must be recognized and carefully monitored. Because of the risk of retrobulbar neuritis, EMB should be used with caution in children who are too young for routine testing of visual acuity. Another possible alternative preventive therapy regimen is PZA plus a fluoroquinolone with reported in vitro activity against M. tuberculosis. A recent report indicates that ofloxacin and cipro- floxacin have similar antimycobacterial potencies in vitro (24). Suggested doses for these agents include 400 mg twice a day of ofloxacin and 750 mg twice a day of ciprofloxacin. Sparfloxacin, another quinolone that has shown good in vitro activity against M. tuberculosis, is under investigation (25). However, to date, sparfloxacin is not licensed in the United States for any indication and would be available only under an investigational new drug (IND) agreement. For all quinolones, in vivo evidence of efficacy against M. tuberculosis is very limited. Short-term administration of quinolones is generally well tolerated, but therapy of 6-12 months' duration has not been studied. Arthropathy has been noted in experiments with immature animals (26); therefore, quinolones should be used in a preventive therapy regimen for children or pregnant females only if the potential benefits justify the potential side effects. Aminoglycosides -- such as SM, KM, and amikacin -- or the polypep- tide antibiotic capreomycin might also be considered for inclusion in an alternative preventive therapy regimen. These drugs are at least partially bactericidal, but they have the disadvantage of requiring injection, creating problems both with logistics and with patient acceptability. The most common serious adverse effects of these drugs are ototoxicity and nephrotoxicity. Para-aminosalicylic acid, ethionamide, and cycloserine are the other antituberculosis drugs sometimes used in the United States. None of these drugs is recommended for use as preventive therapy because of their high frequency of side effects and relatively low efficacy against M. tuberculosis. EVALUATION OF PATIENT OUTCOMES The Clinical Research Branch, Division of Tuberculosis Elimin- ation, CDC, is interested in evaluating the efficacy and toxicity associated with multidrug preventive therapy regimens; the outcomes of persons infected with multidrug-resistant strains of M. tuberculosis who are clinically monitored but who do not take multidrug preventive therapy; and the management and outcomes of patients with MDR-TB. Clinicians and public health officials who are managing patients in the above categories, and those interested in enrolling eligible patients in a pilot study of TB preventive therapy with fluoro- quinolones, are encouraged to call the Clinical Research Branch at (404) 639-2530. References
Figure_1 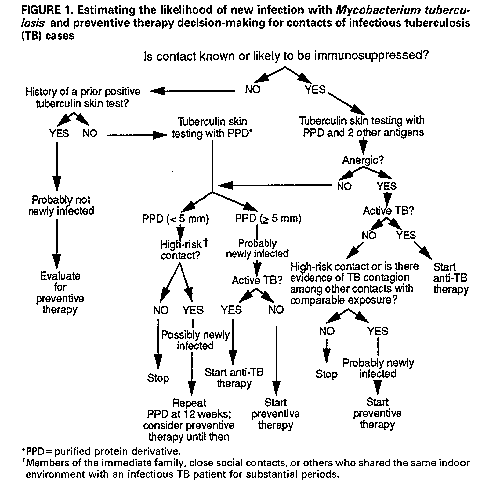 Return to top. Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Likelihood of infection with multidrug-resistant Mycobacterium tubercu-
losis among contacts thought to be newly infected *
=================================================================================================
Estimated likelihood of
Infectiousness Closeness and Contact's risk of infection with
of the source intensity of exposure to drug- multidrug-resistant
MDR-TB + case MDR-TB exposure susceptible TB M. tuberculosis &
------------------------------------------------------------------------------------
+-------------------+
+ + - ş High ş
ş ş
+ - - ş High-intermediate ş
ş ş
- + - ş High-intermediate ş
ş ş
- - - ş Intermediate ş
ş ş
+ + + ş Intermediate ş
+-------------------+
+ - + Low-intermediate
- + + Low-intermediate
- - + Low
------------------------------------------------------------------------------------
Key: (+)=high; (-)=low.
* Anergic contacts should be considered likely to be newly infected if there is evidence of
contagion among contacts with comparable exposure.
+ MDR-TB = multidrug-resistant tuberculosis.
& Multidrug preventive therapy should be considered for persons in high, high-intermediate, and
intermediate categories.
=================================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Risk factors for the development of active tuberculosis among persons
infected with Mycobacterium tuberculosis
===============================================================================================
Estimated increased risk for
tuberculosis compared with
Risk factor persons with no known risk factor
----------------------------------------------------------------------------------
Acquired immunodeficiency syndrome 170.0
Human immunodeficiency virus infection 113.0
Other immunocompromising conditions * 3.6-16.0
Recentness of infection (<=2 years) 15.0
Age of contact (<=5 years and >=60 years) 2.2-5.0
----------------------------------------------------------------------------------
* For example, diabetes mellitus type I, renal failure, carcinoma of head or neck, iatrogenic
immunosuppression.
===============================================================================================
Return to top. Figure_2 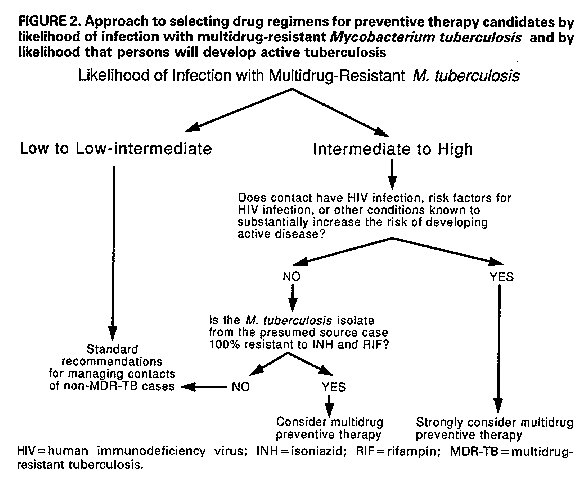 Return to top. Disclaimer All MMWR HTML documents published before January 1993 are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 08/05/98 |
|||||||||
This page last reviewed 5/2/01
|