For Healthcare Professionals
Clinical Features
Measles is an acute viral respiratory illness. It is characterized by a prodrome of fever (as high as 105°F) and malaise, cough, coryza, and conjunctivitis -the three “C”s -, a pathognomonic enanthema (Koplik spots) followed by a maculopapular rash. The rash usually appears about 14 days after a person is exposed. The rash spreads from the head to the trunk to the lower extremities. Patients are considered to be contagious from 4 days before to 4 days after the rash appears. Of note, sometimes immunocompromised patients do not develop the rash.
CDC’s Dr. Jane Seward describes measles clinical features and what to do if a healthcare provider suspects measles, in this 5-minute video.
The Virus
Measles is caused by a single-stranded, enveloped RNA virus with 1 serotype. It is classified as a member of the genus Morbillivirus in the Paramyxoviridae family. Humans are the only natural hosts of measles virus.
Background
In the decade before the live measles vaccine was licensed in 1963, an average of 549,000 measles cases and 495 measles deaths were reported annually in the United States. However, it is likely that, on average, 3 to 4 million people were infected with measles annually; most cases were not reported. Of the reported cases, approximately 48,000 people were hospitalized from measles and 1,000 people developed chronic disability from acute encephalitis caused by measles annually.
Advice for Travelers

Check that your patients 6 months of age or older who will be traveling internationally are protected against measles.
- Travel Notice: Watch (Level 1): Measles in Belgium
- Travel Notice: Watch (Level 1): Measles in Democratic Republic of the Congo
- Travel Notice: Watch (Level 1): Measles in France
- Travel Notice: Watch (Level 1): Measles in Germany
- Travel Notice: Watch (Level 1): Measles in Guinea
- Travel Notice: Watch (Level 1): Measles in Italy
- Travel Notice: Watch (Level 1): Measles in Indonesia
- Travel Notice: Watch (Level 1): Measles in Romania
In 2000, measles was declared eliminated from the United States. Elimination is defined as the absence of endemic measles virus transmission in a defined geographic area, such as a region or country, for 12 months or longer in the presence of a well-performing surveillance system. However measles cases and outbreaks still occur every year in the United States because measles is still commonly transmitted in many parts of the world, including countries in Europe, Asia, the Pacific, and Africa. Worldwide, 36 cases of measles per 1 million persons are reported each year; an estimated 134,200 die each year.
Since 2000, when measles was declared eliminated from the U.S., the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014. The majority of cases have been among people who are not vaccinated against measles. Measles cases in the United States occur as a result of importations by people who were infected while in other countries and from transmission that may occur from those importations. Measles is more likely to spread and cause outbreaks in U.S. communities where groups of people are unvaccinated.
Outbreaks in countries to which Americans often travel can directly contribute to an increase in measles cases in the United States. In recent years, measles importations have come from frequently visited countries, including, but not limited to, England, France, Germany, India, and the Philippines, where large outbreaks were reported.
Complications
Common complications from measles include otitis media, bronchopneumonia, laryngotracheobronchitis, and diarrhea.
Even in previously healthy children, measles can cause serious illness requiring hospitalization.
- One out of every 1,000 measles cases will develop acute encephalitis, which often results in permanent brain damage.
- One or two out of every 1,000 children who become infected with measles will die from respiratory and neurologic complications.
- Subacute sclerosing panencephalitis (SSPE) is a rare, but fatal degenerative disease of the central nervous system characterized by behavioral and intellectual deterioration and seizures that generally develop 7 to 10 years after measles infection.
People at High Risk for Complications
People at high risk for severe illness and complications from measles include:
- Infants and children aged <5 years
- Adults aged >20 years
- Pregnant women
- People with compromised immune systems, such as from leukemia and HIV infection
Transmission
Measles is one of the most contagious of all infectious diseases; approximately 9 out of 10 susceptible persons with close contact to a measles patient will develop measles. The virus is transmitted by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes. Measles virus can remain infectious in the air for up to two hours after an infected person leaves an area.
Diagnosis and Laboratory Testing
Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Laboratory confirmation is essential for all sporadic measles cases and all outbreaks. Detection of measles-specific IgM antibody and measles RNA by real-time polymerase chain reaction (RT-PCR) are the most common methods for confirming measles infection. Healthcare providers should obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients suspected to have measles at first contact with them. Urine samples may also contain virus, and when feasible to do so, collecting both respiratory and urine samples can increase the likelihood of detecting measles virus.
Molecular analysis can also be conducted to determine the genotype of the measles virus. Genotyping is used to map the transmission pathways of measles viruses. The genetic data can help to link or unlink cases and can suggest a source for imported cases. Genotyping is the only way to distinguish between wild-type measles virus infection and a rash caused by a recent measles vaccination.
For more information, see Measles Lab Tools.
Evidence of Immunity
Acceptable presumptive evidence of immunity against measles includes at least one of the following:
- written documentation of adequate vaccination:
- one or more doses of a measles-containing vaccine administered on or after the first birthday for preschool-age children and adults not at high risk
- two doses of measles-containing vaccine for school-age children and adults at high risk, including college students, healthcare personnel, and international travelers
- laboratory evidence of immunity
- laboratory confirmation of measles
- birth before 1957
Healthcare providers should not accept verbal reports of vaccination without written documentation as presumptive evidence of immunity. For additional details about evidence of immunity criteria, see Table 3 in Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP).
Vaccination
Measles can be prevented with measles-containing vaccine, which is primarily administered as the combination measles-mumps-rubella (MMR) vaccine. The combination measles-mumps-rubella-varicella (MMRV) vaccine can be used for children aged 12 months through 12 years for protection against measles, mumps, rubella and varicella. Single-antigen measles vaccine is not available.
One dose of MMR vaccine is approximately 93% effective at preventing measles; two doses are approximately 97% effective. Almost everyone who does not respond to the measles component of the first dose of MMR vaccine at age 12 months or older will respond to the second dose. Therefore, the second dose of MMR is administered to address primary vaccine failure [1]
Vaccine Recommendations
Children
CDC recommends routine childhood immunization for MMR vaccine starting with the first dose at 12 through 15 months of age, and the second dose at 4 through 6 years of age or at least 28 days following the first dose.
Students at post-high school educational institutions
Students at post-high school educational institutions without evidence of measles immunity need two doses of MMR vaccine, with the second dose administered no earlier than 28 days after the first dose.
Adults
People who are born during or after 1957 who do not have evidence of immunity against measles should get at least one dose of MMR vaccine.
International travelers
People 6 months of age or older who will be traveling internationally should be protected against measles. Before travelling internationally,
- Infants 6 through 11 months of age should receive one dose of MMR vaccine
- Children 12 months of age or older should have documentation of two doses of MMR vaccine (the first dose of MMR vaccine should be administered at age 12 months or older; the second dose no earlier than 28 days after the first dose)
- Teenagers and adults born during or after 1957 without evidence of immunity against measles should have documentation of two doses of MMR vaccine, with the second dose administered no earlier than 28 days after the first dose
Healthcare personnel
Healthcare personnel should have documented evidence of immunity against measles, according to the recommendations of the Advisory Committee on Immunization Practices [48 pages].
For more information, see measles vaccination recommendations.
Some people should not get MMR vaccine. For information about contraindications, see who should NOT get vaccinated with MMR vaccine?
Footnote
- CDC. Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2013;62(RR04);1-34.
Post-exposure Prophylaxis
People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered post-exposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). MMR vaccine, if administered within 72 hours of initial measles exposure, or immunoglobulin (IG), if administered within six days of exposure, may provide some protection or modify the clinical course of disease.
MMR vaccine as post-exposure prophylaxis
If MMR vaccine is not administered within 72 hours of exposure as PEP, MMR vaccine should still be offered at any interval following exposure to the disease in order to offer protection from future exposures. People who receive MMR vaccine or IG as PEP should be monitored for signs and symptoms consistent with measles for at least one incubation period.
If many measles cases are occurring among infants younger than 12 months of age, measles vaccination of infants as young as 6 months of age may be used as an outbreak control measure. Note that children vaccinated before their first birthday should be revaccinated when they are 12 through 15 months old and again when they are 4 through 6 years of age.
Except in healthcare settings, unvaccinated people who receive their first dose of MMR vaccine within 72 hours after exposure may return to childcare, school, or work.
Immunoglobulin (IG) as post-exposure prophylaxis
People who are at risk for severe illness and complications from measles, such as infants younger than 12 months of age, pregnant women without evidence of measles immunity, and people with severely compromised immune systems, should receive IG. Intramuscular IG (IGIM) should be given to all infants younger than 12 months of age who have been exposed to measles. For infants aged 6 through 11 months, MMR vaccine can be given in place of IG, if administered within 72 hours of exposure. Because pregnant women might be at higher risk for severe measles and complications, intravenous IG (IGIV) should be administered to pregnant women without evidence of measles immunity who have been exposed to measles. People with severely compromised immune systems who are exposed to measles should receive IGIV regardless of immunologic or vaccination status because they might not be protected by MMR vaccine.
IG should not be used to control measles outbreaks, but rather to reduce the risk for infection and complications in the people receiving it. IGIM can be given to other people who do not have evidence of immunity against measles, but priority should be given to people exposed in settings with intense, prolonged, close contact, such as a household, daycare, or classroom where the risk of transmission is highest.
After receipt of IG, people cannot return to healthcare settings. In other settings, such as childcare, school, or work, factors such as immune status, intense or prolonged contact, and presence of populations at risk, should be taken into consideration before allowing people to return. These factors may decrease the effectiveness of IG or increase the risk of disease and complications depending on the setting to which they are returning.
The recommended dose of IGIM is 0.5 mL/kg of body weight (maximum dose = 15 mL) and the recommended dose of IGIV is 400 mg/kg.
Post-exposure prophylaxis for healthcare personnel
If a healthcare provider without evidence of immunity is exposed to measles, MMR vaccine should be given within 72 hours, or IG should be given within 6 days when available. Exclude healthcare personnel without evidence of immunity from duty from day 5 after first exposure to day 21 after last exposure, regardless of post-exposure vaccine. [2]
Footnote
- Siegel JD, Rhinehart E, Jackson M, Chiarello L, and the Healthcare Infection Control Practices Advisory Committee, 2007 Guidelines for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.
Isolation
Infected people should be isolated for four days after they develop a rash. Healthcare providers should follow respiratory etiquette and airborne precautions in healthcare settings. Regardless of presumptive immunity status, all healthcare staff entering the room should use respiratory protection consistent with airborne infection control precautions (use of an N95 respirator or a respirator with similar effectiveness in preventing airborne transmission). Because of the possibility, albeit low, of MMR vaccine failure in healthcare providers exposed to infected patients, they should all observe airborne precautions in caring for patients with measles. The preferred placement for patients who require airborne precautions is in a single-patient airborne infection isolation room (AIIR).
People without evidence of immunity who have been exempted from measles vaccination for medical, religious, or other reasons and who do not receive appropriate PEP within the appropriate timeframe should be excluded from affected institutions in the outbreak area until 21 days after the onset of rash in the last case of measles.
Treatment
There is no specific antiviral therapy for measles. Medical care is supportive and to help relieve symptoms and address complications such as bacterial infections.
Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day. The recommended age-specific daily doses are
- 50,000 IU for infants younger than 6 months of age
- 100,000 IU for infants 6–11 months of age
- 200,000 IU for children 12 months of age and older
For more information, see page 351 of the World Health Organization measles and vitamin A guidance [12 pages]. Also see Red Book Online: Measles.
Photos
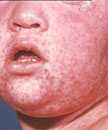
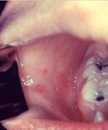
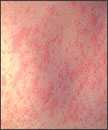
- Photos of Measles and People with Measles
- Immunization Action Coalition
Resources
- Measles 2015: Situational Update, Clinical Guidance, and Vaccination Recommendations
COCA Call/Webinar presented February 19, 2015 - Provider Resources for Vaccine Conversations with Parents
- An Introduction to Measles Powerpoint presentation [20 pages]
- Measles Data and Statistics Powerpoint presentation [15 pages]
- Page last reviewed: February 13, 2017
- Page last updated: July 12, 2017
- Content source:


 ShareCompartir
ShareCompartir