Processing Environmental Samples: CDC Laboratory Protocol
On this Page
Printer-friendly version of this page [3.61 MB, 15 pages]
This manual describes the procedures currently employed by the Centers for Disease Control and Prevention to process environmental samples obtained during investigations of legionellosis outbreaks. It includes information of the collection and concentration of water samples, preparation of samples for bacteriologic examination, formulas for media, and sources for reagents.
National Center for Infectious Diseases
Division of Bacterial and Mycotic Diseases
Respiratory Disease Laboratory Section
January 2005
U.S. Department of Health and Human Services
Public Health Service
Centers for Disease Control and Prevention (CDC)
Atlanta, GA 30333
Introduction
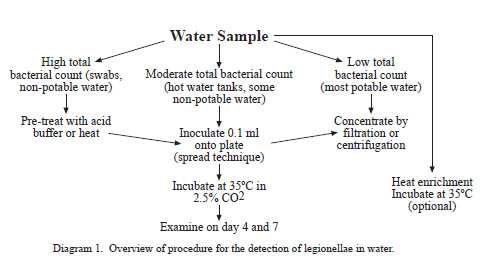
Illness caused by the gram-negative bacteria in the genus Legionella is referred to as legionellosis. Legionellosis consists of two distinct clinical syndromes, Legionnaires’ disease and Pontiac fever. Legionnaires’ disease is characterized by pneumonia where as Pontiac fever is a self-limiting, nonpneumonic, influenza-like illness. Inhalation of aerosols containing the bacterium is presumed to be the primary means of acquiring legionellosis. Aerosolized waters from cooling towers, evaporative condensers, showers, and humidifiers have been identified as sources of infection. Legionella species have been recovered from a wide variety of domestic water systems and are ubiquitous in freshwater environments. Although once considered transient contaminants of natural and domestic waters, legionellae are now known to be free-living organisms surviving as natural components of freshwater ecosystems. Domestic systems are complex environments in which concentrations of legionellae can fluctuate considerably depending upon water temperature, biocide levels, and presence of natural hosts (i.e. protozoa) for legionellae to parasitize. The choice of procedure used to recover legionellae from water samples is dependant upon the expected degree of bacterial contamination in a particular water source. Potable waters generally have low bacterial densities and are either cultured directly or concentrated to detect legionellae. Nonpotable waters, such as those from cooling towers, generally do not require concentration because of their high bacterial concentrations.

Figure 1. Nucleopore filter funnel assembly for concentrating water samples.
This manual describes the procedures currently employed by the Centers for Disease Control and Prevention to process environmental samples obtained during investigations of legionellosis outbreaks. It includes information of the collection and concentration of water samples, preparation of samples for bacteriologic examination, formulas for media, sources of reagents, and air sampling techniques.
Collection of Specimens
An environmental sampling protocol addressing selection of the appropriate sites to sample has been previously published (Barbaree et. al, 1987).Whenever possible, a collection of 1 (one) liter of water is preferred. Larger volumes of water (1 to 10 liters) are occasionally needed to detect legionellae in water that has very low concentrations of these bacteria such as municipal water supplies. If a liter cannot be collected from a sample source, a smaller volume is acceptable. Water samples should be collected in a sterile 1-liter wide-mouth screw cap polypropylene plastic bottle. If the water source has been recently treated with chlorine, add 0.5 ml of 0.1N sodium thiosulfate to each 1 liter sample to neutralize the disinfectant.
Swabs of faucet aerators and shower heads should be taken before water samples from these sites. The sample should be taken with the aerator or shower head removed if possible. Polyester swabs with wooden shafts work well for this purpose. The swabs should be submerged in 3-5 ml of water taken at the same time to prevent drying during transport.
All samples should be transported to the laboratory in insulated coolers as protection against extreme heat or cold. Samples that will not reach the laboratory within 72 hrs should be refrigerated before shipping. Samples that reach the laboratory but cannot be processed within 72 hrs of collection should be refrigerated.
Top of PagePreparation of Specimens for Bacteriologic Examination
a) Filtration (potable water)
Samples are filter concentrated in a biological safety cabinet by pouring the samples into a sterile 47-mm filter funnel assembly containing a 0.2um polycarbonate filter (Fisher Scientific, 3970 Johns Creek Ct., Suite 500, Suwanee, GA 30024). A vacuum source and side-arm flask are necessary to operate the apparatus (Figure 1). Care should be taken to avoid drying of the filters. When the sample has passed through the filter, the filter is removed aseptically from the holder with sterile filter forceps, folded to the outside, and placed into a sterile, 50-ml centrifuge tube containing 5ml of sterile water. The centrifuge tube is then vortexed for one minute to free bacteria and organic material from the filter. If more than one filter is required to concentrate a sample, the additional filters are added to the same sample tube.
b) Direct Plating (nonpotable water)
Nonpotable water rarely requires concentration and can be processed directly. Samples that are found to have high concentrations of bacteria upon direct plating can be treated with a low pH buffer or serially diluted with sterile deionized water, an appropriate buffer, or sterile chorine-free tap water to improve primary isolation of legionellae.
 Top of Page
Top of Page
Plating of Specimens
Buffered charcoal yeast extract (BCYE) agar containing 0.1% alpha-ketoglutarate is the base medium used for the recovery of Legionella from environmental and clinical specimens (Appendix 1). Two types of selective BCYE agar supplemented with antimicrobial agents are included in the standard regimen of media used in the processing of environmental samples. The first, designated PCV, contains polymyxin B, cycloheximide, and vancomycin. The second, GPCV, is identical to PCV except for the addition of the amino acid glycine to the medium. PCV without L-cysteine [PCV(-)] may be used as a negative control medium. Several variations in the antimicrobial supplements have been described and appear to be adequate for the recovery of legionellae. Figure 2 shows a water sample cultured on the four media described above.
Although the majority of Legionella spp. grow readily on BCYE, some require supplementation with bovine serum albumin to enhance growth. Legionella micdadei and several strains of Legionella bozemanii show a preference for BCYE with 1.0% albumin (ABCYE) over standard BCYE. The inclusion of ABCYE in the regimen of plating media may be beneficial when clinical evidence indicates that a species other than Legionella pneumophila may be the causative agent.
Plating of Filtered Samples
- After vortexing of filter (see Preparation of Specimens), inoculate 1 BCYE, 2 PCV, and 2 GPCV and 1 PCV (-) plate each with 0.1 ml of the suspension and spread with a sterile glass or sterile disposable plastic rod.
- Incubate plates at 35ºC in a candle jar or in a humidified incubator with an atmosphere of 2.5% CO2 in air.
- Store the remainder of the sample at 4ºC.
Direct Plating
- Place 0.1 ml of the sample onto BCYE and modified BCYE plates as described above and spread with a sterile glass rod or sterile disposable plastic spreader.
- Incubate plates as described above.
Acid Treatment
For some water samples with high concentrations of bacteria, it is necessary to use a selective procedure to reduce the number of non-Legionella bacteria before culture. Acid treatment and heating of samples are routinely used for this purpose. Legionellae are more resistant to lower pH and brief exposure to higher temperatures than many other freshwater bacteria. The following procedure is used by CDC laboratories to prepare these samples for culture.
- Place 1.0 ml of the vortexed suspension into a sterile 15 ml centrifuge tube containing 1.0 ml of acid buffer and mix (Appendix 2).
- Incubate the acidified suspension for 15 minutes at room temperature. (Acid treatment can be extended to 30 minutes if plates are overgrown after initial 15-minutes treatment).
- Place 0.1 ml of the suspension onto BCYE and modified BCYE plates and spread with a sterile glass rod or sterile disposable plastic spreader.
- Incubate plates at 35ºC in a candle jar or in a humidified incubator with an atmosphere of 2.5% CO2 in air.
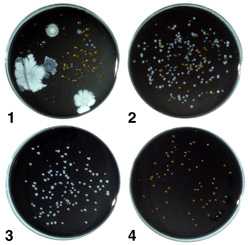
Figure 2. A water sample containing legionellae after culture on the four types of media. Plate 1, BCYE agar with numerous non-Legionella bacteria and a few Legionella colonies; Plate 2, PCV agar with numerous Legionella colonies and other bacteria; Plate 3, GPCV agar with Legionella, few, if any non-Legionella bacteria are present; Plate 4, PCV-without cysteine agar with some non-Legionella bacteria; no legionellae are present.
Examination of Cultures for Legionellae
- Examine all cultures after 72 to 96 hours incubation. Macroscopic examination should be done with a dissecting microscope and oblique lighting to detect bacterial colonies resembling Legionella. These colonies are convex and round with entire edges (Figure 3). The center of the colony is usually bright white in color with a textured appearance that has been described as “cut-glass like” or speckled. The white center of the colony is often bordered with blue, purple, green or red autofluorescence. Further examination of primary isolation plates with long-wave UV light can easily detect these fluorescent colonies.
- Aseptically pick each suspect colony onto (a) BCYE agar plate without L-cysteine [BCYE(-)] or a BCYE/BCYE(-) biplate. If a L-cysteine (-) biplate is not available, a less desireable alternative is another L-cysteine media such a blood agar plate. Pick 2-4 representative colonies per water sample
- Streak the inoculated portion of each plate with a sterile loop to provide areas of heavy growth, and incubate the plates for 24 hrs.
- If no growth is detected after 24 hrs, continue incubation for an additional 24 hrs. Colony picks that grow on BCYE agar, but not BCYE(-) agar, are presumptive Legionella species and must be examined further for definite identification using the direct fluorescent antibody (DFA) or slide agglutination test (SAT).
- Since Legionella is a relatively slow-growing bacterium, negative plates should be reincubated until 7 days postinoculation and then re-examined for Legionella colonies. Discard plates after the seventh day.
Heat Enrichment
Heat enrichment (or “coculture”) of specimens that are negative for Legionella upon primary culture can improve recovery of this organism significantly. This procedure is based on the finding that many water samples contain protozoa that can serve as host for the intracellular multiplication of legionellae. Incubating the samples at 35ºC allows the legionellae to multiply in these protozoa and achieve a culturable concentration. Results from several epidemic investigations indicate that subsequent replating of specimens results in the isolation of Legionella spp. from approximately 30% of all samples negative upon primary isolation. Twenty-five milliliter aliquots of samples are incubated at 35ºC in 50 ml plastic screw cap centrifuge tubes. With swab specimens, the entire swab sample is incubated. The tube caps should be tightened to minimize evaporation of the accompanying 3-5 ml of water sample. Incubated samples are cultured at 2-week intervals for as long as 6 weeks. Samples are plated on the same regimen of plates as in primary isolation. If overgrowth of media occurs, samples can be acid treated as previously described.
Top of PageAir Sampling
In certain circumstances, it is beneficial to demonstrate the presence of legionellae in aerosol droplets associated with suspected reservoirs of the bacterium. Although not essential for the identification of reservoirs of disease-causing strains of Legionella, air sampling can better define the role of certain devices in disease transmission.
Sampling for biological contaminants in air can be used to determine the presence of a particular organism in an air sample, the number of viable organisms, the number of particles bearing organisms, and the size distribution of particles. Before initiating a sampling protocol, an investigator should determine which information will be collected. For each determination, the investigator should consider the type of sampler to be used, the probable concentration of organisms, sampling time, and environmental factors affecting the aerosol.
When obtaining air samples for Legionella, a primary objective is establishing the presence of the bacteria in aerosol droplets. The following methods are directed toward this purpose. Although some information regarding particle size and numbers of viable bacteria can be calculated from these procedures, this information should be considered approximate.
The basic methods for sampling airborne bacteria include impingment in liquids, impaction on solid surfaces, filtration, sedimentation, centrifugation, and precipation. Two methods that have been used to sample for legionellae will be described:
- impingement in liquid using an all-glass impinger; and
- impaction on solid media using an Anderson sampler. Although other methods have been used to successfully sample for legionellae, these two are most frequently successful in our field investigation.

Figure 3. Legionella colonies as seen through a dissecting microscope on primary isolation (4 days incubation). Note the white "cut-glass" appearance of the center of the colony and the purple iridescence which borders it. The iridescence can be one of several colors; the significance of the color is unknown.
Flowmeter and Vacuum Source
Several configurations of air sampling equipment can be used to obtain a quantitative sample. The method described here uses a calibrated flowmeter connected in the vacuum line between the sampler and vacuum source. The flowmeter is calibrated using a calibrating flowmeter. Ideally, flowmeters should be calibrated on site before each investigation. The desired flow rate can be obtained using the adjustment screw on the vacuum pump. The tubing used to connect all equipment should be uniform size and the length of this tubing should be kept to a minimum.
All-Glass Impinger (Chemical Corps Type)
An all-glass impinger (AGI) is shown in Figure 4. This is a Chemical Corps type high velocity impinger with the stem 30 mm from the bottom of the flask. This distance is preferred for the collection of viable vegetative cells. These samplers use the principle of impingement and washing of air, in which organisms are entrapped in a liquid medium. Because of the velocity at which samples are collected, clumps tend to be fragmented, leading to a more accurate count of bacteria present in the air. The disadvantages are that this velocity tends to destroy some vegetative cells. Also, AGIs are easily broken in the field.
Procedure for Using the AGI
- Autoclave AGIs before use.
- Aseptically fill each AGI with 20 ml of sterile 0.25% yeast extract broth immediately before use.
- Securely position the AGI and connect via vacuum tubing to the calibrated flow meter and vacuum pump as shown in Figure 4.
- Turn on the vacuum pump and adjust the flow rate to 6.0 liters per minute.
- If the sample is to be an aerosol from a shower, faucet, etc., immediately turn on the water after the flow rate is adjusted.
- Sample for 10 to 30 minutes, aseptically remove the yeast extract broth, and transfer to a sterile tube.
- Process the yeast extract broth as a water sample as previously described.
Andersen Sampler
A two-stage and six-stage Andersen sampler are shown in Figure 5. BCYE agar plates, with or without selective agents, are placed between each stage before each use. Andersen samplers are viable particle samplers in which particles pass through jet orifices of increasingly smaller size in cascade fashion until they impact on an agar surface. The agar plates are then removed and incubated in order to culture any legionellae present. The stage distribution of the legionellae should indicate the extent to which the bacteria would have penetrated the respiratory system.
Anderson samplers are preferred in the field because of their durability and the fact that no further manipulations of the sample are necessary to detect the legionellae.
Andersen Sampling Scheme
Two samplers should be run simultaneously, one containing non selective BCYE agar, the other containing GPCV agar. If the sample is an aerosol from a shower, faucet, etc., a background check should be preformed with the water source turned off. Two-stage samplers containing BCYE and GPCV agar plates are run for 15 minutes for this background sample.
Two six-stage samplers are used for all samples other than background. These samples are collected for 30 minutes.
Anderson Procedure
- Clean each stage with a disinfectant such as isopropyl alcohol, and allow to dry thoroughly.
- Carefully place agar plates between each stage and check O rings to ensure that a proper seal is maintained. Silicone stopcock grease may be needed to help hold O rings in place.
- Tighten thumbscrews and cover the orifice of the top stage with a Petri dish lid until use.
- Position the Andersen sampler and connect via tubing to the calibrated flow meter and vacuum pump as shown in Figure 5.
- Remove Petri dish lid, turn on the vacuum pump, and adjust the flow rate to 28.3 liters per minute.
- Turn on the water source immediately and collect the sample for 30 minutes.
- Remove the agar plates and label each to indicate the stage number and incubate as soon as possible. If the sample must be shipped to a laboratory, they should be packed and shipped without refrigeration.
- Inspect plates for the presence of legionellae as previously described.
General Considerations and Safety
Samplers should be placed in a location representative of human exposure. For example, when sampling a shower, the samplers should be placed at head level. Often it is convenient to place the samplers, flow meters, and vacuum pumps on a step ladder to collect such samples. A heavy duty extension cord should be included with supplies for field studies. When sampling near water sources, care should be taken to avoid electric shock. An investigator should wear an OSHA-approved respirator if sampling involves exposure to potentially infectious aerosols.
Appendix 1
Preparation of Media
Buffered charcoal yeast extract (BCYE) agar
A. Composition
- Norit SG charcoal
- Yeast extract
- ACES buffer
- Ferric pyrophosphate (soluble)
- L-cysteine HCl-H20
- Agar, bacto (Oxoid)
- Potassium alpha-ketoglutarate
- Distilled water
- 2.0 g
- 10.0 g
- 10.0 g
- 0.25 g
- 0.40 g
- 17.0 g
- 1.0 g
- 1,000 ml
Antibiodic components for media
- 1. GPCV medium
- Glycine
- Polymyxin B
- Vancomycin
- Cycloheximide
- 2. PCV medium – GPCV medium without glycine
- 0.3%
- 100 units/ml
- 5 ug/ml
- 80 ug/ml
ABCYE medium
- Bovine serum albumin (fraction V)
- 10g/L
Dehydrated BCYE agar base is available from several commercial sources.
B. Directions
- Add ACES buffer to 940 ml of distilled water and dissolved in a 50ºC water bath.
- Add enough 1.0 N KOH (about 40 ml) to the buffer solution to bring the pH up to 6.9 and mix. (Do not use NaOH because it has been found to be inhibitory to Legionella pneumophila).
- Into a second flask, add charcoal, yeast extract, alpha-keto-glutarate, and agar. Mix the dry powders.
- Pour the buffer solution into the second flask containing the dry powders and mix.
- Carefully heat to dissolve the agar, then sterilize by autoclaving at 121ºC for 15 minutes.
- Immediately place the medium in 50ºC water bath.
- For complete medium, add membrane-filtered solution of L-cysteine to the medium and mix thoroughly.
- Add membrane-filtered soluble ferric pyrophosphate, and mix thoroughly. Do not mix iron and cysteine before adding to medium.
- Adjust the pH of the medium to 6.9 at room temperature.
Since reagents may vary, each laboratory must determine the amount of KOH required. Hold the completed medium at 50ºC, pour a 10 ml sample, and check the pH at room temperature. When necessary, adjust the completed medium with either 1.0 N KOH or 1.0 HCl. Note that the pKa of ACES buffer is 6.9 at 20ºC and 6.8 at 25ºC. Its pKa is affected by temperature (0.02 pH unit/o). However, once the agar has solidified, the pH does not appear to change with temperature but remains at 6.9.
- For preparation of BCYE + antibiodics, add membrane-filtered antibiodics and mix.
- For BCYE + albumin agar, dissolve the albumin in distilled water and filter sterilize before addition to the medium.
- Dispense 20 ml per 15 X 100-mm Petri dish.
The medium must be mixed frequently during the pouring to keep the charcoal particles suspended. After the medium has solidified, the plates should be stored in plastic bags in the refrigerator in the dark. They should be good for approximately 4 months, provided they pass quality control.
C. Precautions
- The soluble ferric pyrophosphate must be kept dry and in the dark.
- Fresh solutions of L-cysteine and ferric pyrophosphate must be prepared each time. Since L-cysteine is a chelating agent, it must be added to the medium first. Both solutions must be added slowly. This is also true for adding the 1.0 N KOH.
- Do not use ferric pyrophosphate if its green color becomes yellow or brown.
Quality Control of Media
Each batch of medium should be tested for pH and the ability to support the growth of L. pneumophila. Ideally, all media should be tested with a standardized tissue inoculum. However, since this material is not generally available, stock culture inoculum is recommended.
- The pH of the medium must be 6.90 +/- 0.05 Measure with a pH meter. To check solid media, use a surface electrode (if available), or emulsify the agar from one plate in distilled water, and measure the pH of the emulsion. The pH is critical. If given lots of ingredients do not conform to the acceptable pH range, adjust the pH of the completed liquid medium by adding 1.0 N KOH or 1.0 N HCl.
- Check for support of growth in the following manner:
- Prepare a standard stock culture inoculum by suspending L. pneumophila (log phase) in sterile distilled water to a concentration of 10º cells/ml.
- Inoculate the medium with 0.05 ml of the standard inoculum. Streak the plates to obtain isolated colonies, and incubate at 35ºC in air plus 2.5% CO2.
- Examine the media daily. On agar plates, growth should be present in the heavily inoculated area after one day. Isolated colonies should be macroscopically visible in 2 to 3 days. If cells in standard inoculum have been refrigerated or frozen, growth will be slower.
- If possible, selective media should be checked using an environmental water specimen containing a known concentration of legionellae and other bacteria.
Appendix 2
Reagents for Acid Treatment Procedure
KCl-HCl Acid
- 0.2 M KCl (14.9 g/L in distilled water)
- 0.2 M HCl (16.7 g/L in distilled water)
- Mix 18 parts of (A) with 1 part of (B)
- Dispense into screw-capped tubes in 1.0 ml volumes and sterilze by autoclaving
References
- Barabee, J.M., G.W. Gorman, W.T. Martin, B.S. Fields, and W.E. Morrill. 1987. Protocol for sampling environmental sites for legionellae. Appl Environ Microbiol. 53:1454-1458.
- Bopp, C.A., J.W. Summer, G.K. Morris, and J.G. Wells. 1981. Isolation of Legionella spp. from environmental water samples by low-pH treatment and use of a selective medium. J Clin Microbiol. 13:714-719.
- Breiman, R.F., B.S. Fields, G.N. Sanden, L. Volmer, and J.S. Spika. 1990. Association of shower use with Legionnaires’ disease: possible role of amoebae. JAMA 263:2924-2926.
- Breimen, R.F, W. Cozen, B.S. Fields, T.D. Mastro, S.J. Carr, J.S. Spika, and L. Mascola. 1990. Role of air sampling in investigation of an outbreak of Legionnaires’ disease associated with exposure to aerosols from an evaporative condenser. J Infect Dis. 161:1257-1261.
- Edelstein, P.H., 1981. Improved semi-selective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 14:298-303.
- Edelstein, P.H., and M.A. Edelstein. 1991. Comparison of different agars used in the formulation buffered charcoal yeast extract medium. J Clin Microbiol. 29:190-191.
- Feeley, J.C., R.J. Gibson, G.W. Gorman, N.C. Langford, J.K. Rasheed, D.C. Mackel, and W.B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 10:437-441.
- Fields, B.S. 2002. Legionellae and Legionnaires’ Disease. p. 860-870. In Hurst, C.J., R.L. Crawford, G.R. Knudsen, M.J. McInerney, and L.D. Stetzenbach (ed.) Manual of Environmental Microbiology (2 ed.) ASM Press, Washington, D.C.
- Fields, B.S., G.N. Sanden, J. M. Barbaree, W.E. Morrill, R.M. Wadowsky, E.H. White, and J.C. Feeley. 1989. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr Microbiol. 18:131-137.1
- Fields, B.S. J.M. Barbaree, G. N. Sanden, and W. E. Morrill. 1990. Virulence of Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect Immun. 58:3139-3142.
- Morrill, W.E., J.M. Barbaree, B.S. Fields, G.N. Sanden, and W.T. Martin. 1990. Increased recovery of Legionella micdadei and Legionella bozemanii on buffered charcoal yeast extract agar supplemented with albumin. J Clin Microbiol. 28:616-618.1
- Pasculle, A.W., J.C. Feeley, R.J. Gibson, L.G. Cordes, R.L. Myerowitz, C.M. Patton, G.W. Gorman, C.L. Carmack, J.W. Ezzell, and J.N. Dowling. 1980. Pittsburg pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 141(6):727-732.
- Sanden, G.N., W.E. Morrill, B.S. Fields, R.T. Breiman and J.M. Barbaree. 1992. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl Environ Microbiol. 58 (6):2001-2004.
- Wadowsky, R.M., and R.B. Yee. 1981. Glycine-containing selective medium for isolation of Legionellaceae from environmental specimens. Appl Environ Microbiol. 42:768-772.
- Wilkinson, H.W., 1987. Hospital-laboratory diagnosis of Legionella infections. p.5-7. Centers for Disease Control, Atlanta, GA
- Wolf, H.W., P. Skaily, L.B. Hall, M.M. Harris, H.M. Decker, L.M. Buchanan and C.M. Dahlgren. 1964. Sampling Microbiological Aerosols. Public Health Service Publication No. 686. Government Printing Office. Washington, DC.
- Page last reviewed: February 5, 2013
- Page last updated: August 9, 2016
- Content source:


 ShareCompartir
ShareCompartir

