Reporting Patient Safety Events Associated with EHRs
ONC: The Future of Health Care: Electronic Health Records
|
[3 mins, 30 sec] July 18, 2011Healthcare organizations are encouraged to report instances in which poor EHR design, configuration or implementation resulted or could have resulted (“near-miss”) in an adverse patient outcome. More credible data is needed to identify specific patient safety risks so that recommended practices can be developed and integrated into IT standards, as noted in the November 2011 report from the Institute of Medicine, Health IT and Patient Safety: Building Safer Systems for Better Care. Depending on the issue, healthcare organizations can report patient safety-related concerns to one or more of the following: the EHR Technology Developer, the ONC-Authorized Certification Body, an AHRQ approved Patient Safety Organization and the FDA. Also, the FDA, ONC, and FCC have proposed a Health IT Safety Center that could become an option for EHR and health IT patient safety event reporting in the future. See the tabs below for more information regarding the various reporting options. |
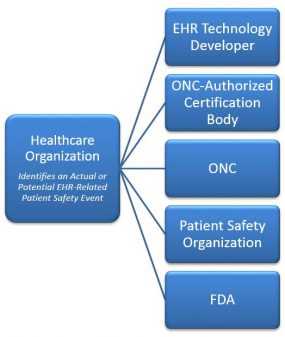
Reporting Options for Actual or Potential (near-miss) EHR-Related Patient Safety Events. |
Safety-related concerns should be reported to the EHR Technology Developer so they may monitor the performance of their product and evaluate if an EHR-based solution is needed to prevent recurrence. The ONC HIT Certification Program’s Program Policy Guidance #13-01 supports end-user reporting to the EHR Technology Developer that developed the full EHR or module. The ONC-Authorized Certification Bodies (ONC-ACBs) must also ensure the EHR Technology Developer implements a complaint process for safety-related capabilities.
The list of certified EHR products is located on the ONC’s website: https://chpl.healthit.gov/#/search
EHR certification bodies are authorized by the Office of the National Coordinator to perform Complete EHR and EHR Module testing and certification. The ONC HIT Certification Program’s Program Policy Guidance #13-01 supports end-user reporting of patient safety-related concerns for certified products and capabilities to the ONC-Authorized Certification Body that certified the particular EHR or EHR module impacted. The guidance further supports annual surveillance reporting of the safety-related data collected by the ONC-ACBs to the ONC. Their websites are provided below.
The certifying body for a specific EHR can be searched on the ONC’s website at: https://chpl.healthit.gov/#/search
Submit a health IT concern to ONC
The Office of the National Coordinator oversees an online form where users can log complaints about certified health IT products so that ONC staff can better triage, track, route and respond to health IT concerns and challenges. The ONC recommends first contacting the developer or vendor, then the ONC-Authorized Certification Body (ACB), which should be able to work with you and the developer to resolve most issues. If the issue remains unresolved, then submit your issues to the ONC. Click the button above or this link: http://www.HealthIT.gov/healthitcomplaints
The ONC wants to know about:
- Challenge related to data blocking (“when someone or an entity knowingly and unreasonably interferes with the exchange or use of health information”)
- Inability for EHRs to share or receive health information
- Usability issues
- Failure for certified products to perform as expected
Healthcare organizations can voluntarily report concerns to a Patient Safety Organization (PSO) approved by the Agency for Healthcare Research and Quality (AHRQ). Communications with PSOs are afforded privilege and confidentiality protections under the Patient Safety Act to encourage reporting and allay fears of legal discovery related to the collection and analysis of patient safety event data.
For more information, see the Patient Safety Organization Homepage at AHRQ, including:
- Frequently Asked Questions
- How to choose a PSO
- Federally-Listed Patient Safety Organizations
EHR functionalities (whether certified or non-certified) are not classified by the FDA within a formal medical device class. The FDA is exercising broad enforcement discretion related to EHR functionality and does accept reports of EHR related patient safety events.
Certain serious patient safety events associated with medical devices are required to be reported to the FDA, such as those resulting in death or disability. The FDA also accepts voluntary reporting of medical device-related patient safety concerns that do not result in a serious patient safety event. Reports submitted to the FDA are published in the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database.
For more information, see the following links to the U.S. Food & Drug Administration:
- What is a serious adverse event?
- Medical Device Reporting
- Manufacturer and User Facility Device Experience (MAUDE) database
Note: On April 3, 2014, the ONC, FDA and FCC released a report proposing a strategy and recommendations for a risk-based framework for health IT. The proposal includes creation of a public-private Health IT Safety Center by the ONC in collaboration with the FDA, the FCC, HHS’ Agency for Healthcare Research and Quality (AHRQ) and other stakeholders. Implementation of the strategies and recommendations in this report may create alternative approaches in the future for reporting and resolving patient safety events associated with EHRs and other health IT.
HHS Press Release: FDASIA Health IT Report – Proposed Strategy and Recommendations for a Risk-Based Framework
The Joint Commission – ONC funded investigational health IT work
The ONC awarded a contract to The Joint Commission to expand its capacity to investigate the role of health IT as a cause of adverse events, and will develop education materials and training opportunities to enable healthcare providers to investigate and analyze health IT-related adverse events and develop follow-up and corrective action. Access to some of these materials is provided here.
Safe Health IT Saves Lives
- Podcast: Safe Use of Health IT [4:01 mins]
- Video: Safe Health IT Saves Lives [2:26 mins]
- Online Education Course (free): Investigating and Preventing Health IT – Related Patient Safety Events
- Reports:
References
- Health IT Safety Center Roadmap (ONC contract with RTI)
- ONC Fact Sheet: Health IT Patient Safety Action and Surveillance Plan
- The Joint Commission ONC Contract fact page
- The Joint Commission: Report a Sentinel Events and complaints of all kinds associated with a healthcare organization
- The Partnership for Health IT Patient Safety (ECRI)
References
ONC Program Policy Guidance #13-01: Authorized Certification Body Annual Surveillance Plan
ONC Publication: How to Identify and Address Unsafe Conditions Associated with Health IT
This guide aims to help healthcare organizations and Patient Safety Organizations improve reporting of unsafe conditions associated with EHRs and other health IT.
- Page last reviewed: May 18, 2017
- Page last updated: May 18, 2017
- Content source:


 ShareCompartir
ShareCompartir