Content on this page was developed during the 2009-2010 H1N1 pandemic and has not been updated.
- The H1N1 virus that caused that pandemic is now a regular human flu virus and continues to circulate seasonally worldwide.
- The English language content on this website is being archived for historic and reference purposes only.
- For current, updated information on seasonal flu, including information about H1N1, see the CDC Seasonal Flu website.
Questions and Answers: 2009 H1N1 and Pneumococcal Disease in the News
November 25, 2009, 2:00 PM ET
- What is invasive pneumococcal disease?
- What does CDC know about invasive pneumococcal disease among people who get 2009 H1N1 or seasonal influenza?
- What is Active Bacterial Core surveillance (ABCs)?
- What percentage of people hospitalized with 2009 H1N1 flu have developed invasive pneumococcal disease?
- Is 2009 H1N1 flu associated with an increase in invasive pneumococcal disease?
- What, so far, does the data from Colorado show?
- If the increase in invasive pneumococcal disease is related to 2009 H1N1 flu, is this expected?
- Do the preliminary data from Colorado follow similar trends to flu pandemics that occurred in the twentieth century?
- What vaccines are available to help prevent invasive pneumococcal disease and flu infections?
- Could the cases of invasive pneumococcal disease in Colorado have been prevented with pneumococcal vaccines?
- What percentage of adults with high risk conditions has received the pneumococcal polysaccharide vaccine?
- How is invasive pneumococcal disease diagnosed in adults?
- How is invasive pneumococcal disease treated in adults during flu season?
- Where can I find more information about invasive pneumococcal disease, pneumococcal vaccines, and flu vaccines?
2009 H1N1 and Seasonal Influenza Infections and Invasive Pneumococcal Disease
What is invasive pneumococcal disease?
Invasive pneumococcal disease (IPD) is an infection caused by a type of bacteria called Streptococcus pneumoniae (pneumococcus). Invasive disease means that germs invade parts of the body that are normally free from germs, like blood or spinal fluid. When this happens, disease is usually very severe, causing hospitalization or even death. When pneumococcal bacteria invade the lungs, they can cause pneumonia. They can also invade the bloodstream, causing bacteremia, and/or the tissues and fluids surrounding the brain and spinal cord, causing meningitis.
What does CDC know about invasive pneumococcal disease among people who get 2009 H1N1 or seasonal influenza?
Influenza (flu) infections can make people more likely to develop pneumococcal infections. Pneumococcal infections are a complication of 2009 H1N1 and seasonal flu infections and can cause serious complications, including pneumonia and death. CDC tracks pneumococcal disease through Active Bacterial Core surveillance (ABCs), part of the Emerging Infections Program Network (EIP).
What is Active Bacterial Core surveillance (ABCs)?
ABCs is an active laboratory- and population-based system for tracking important invasive bacterial pathogens (germs). For each case of invasive disease in the surveillance population, a case report with basic demographic information (age, sex, ethnicity, etc.) is completed and bacterial isolates are sent to CDC and other reference laboratories for additional laboratory testing. ABCs currently operates in 10 areas across the United States, representing a population of over 38 million persons.
What percentage of people hospitalized with 2009 H1N1 flu have developed invasive pneumococcal disease?
We do not know the exact percentage. Some people who have 2009 H1N1 flu will develop invasive pneumococcal disease (IPD); those who do may not develop symptoms of IPD until several days or even a week after they experience flu symptoms. Because of this timing and because not everyone is tested for both infections, we don’t know what percentage of people hospitalized with 2009 H1N1 flu go on to develop IPD.
Is 2009 H1N1 flu associated with an increase in invasive pneumococcal disease?
Possibly. Some ABCs sites have seen greater than expected numbers of cases of invasive pneumococcal disease (IPD) coincident with increases in laboratory confirmed influenza-associated hospitalizations. CDC has been working with state and local public health officials in Colorado, an ABCs site, to collect additional data on IPD cases there. For a graphical representation of this data, please see the chart below; other ABCs sites are experiencing similar trends. Findings are preliminary and may change with further investigation.
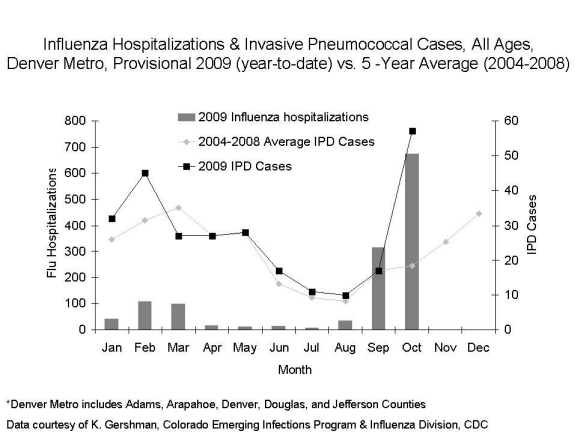
What, so far, does the data from Colorado show?
There is good evidence that 2009 H1N1 flu may be responsible for the increase in invasive pneumococcal disease (IPD) cases in the Denver Metro area (5-year average number of cases in October, ~20; total number in October 2009, 58). The increase in pneumococcal disease in the Denver Metro area was coincident with the October peak of the 2009 H1N1 flu wave there. Ten patients of 48 patients with IPD who were tested for flu were positive for flu, including 32 tested using the reverse transcriptatse polymerase chain reaction (RT-PCR) test. We believe others likely had preceding influenza, but the timing of their testing made it challenging to document influenza.
If the increase in invasive pneumococcal disease is related to 2009 H1N1 flu, is this expected?
Yes. Increases in pneumococcal disease were reported during all three of the flu pandemics that occurred in the twentieth century. A key difference is that now we have two pneumococcal vaccines that may help to prevent these infections.
Do the preliminary data from Colorado follow similar trends to flu pandemics that occurred in the twentieth century?
Yes. An increase in invasive pneumococcal disease (IPD) cases as was seen in the Denver Metro area has also been seen in prior pandemics.
What vaccines are available to help prevent invasive pneumococcal disease and flu infections?
For the prevention of pneumococcal disease, two vaccines are currently available in the United States.
- All children less than 5 years of age should receive pneumococcal conjugate vaccine (PCV7) according to current recommendations.
- In addition, the 23-valent pneumococcal polysaccharide vaccine (PPSV) should be administered to all persons 2-64 years of age with high risk conditions and everyone 65 years and older.
- Special emphasis should be placed on vaccinating adults under 65 years of age who have established high-risk conditions for pneumococcal disease; PPSV coverage among this group is low and this group may be more likely to develop secondary bacterial pneumonia after a flu infection.
- For those 19 through 64 years of age, high-risk conditions include: having asthma or smoking cigarettes.
- For those 2 through 64 years of age, high-risk conditions include: chronic cardiovascular disease (congestive heart failure and cardiomyopathies), chronic pulmonary disease (including chronic obstructive pulmonary disease and emphysema), diabetes mellitus, alcoholism, chronic liver disease (including cirrhosis), cerebrospinal fluid leaks, cochlear implant, functional or anatomic asplenia including sickle cell disease and splenectomy, immunocompromising conditions including HIV infection, leukemia, lymphoma, Hodgkin’s disease, multiple myeloma, generalized malignancy, chronic renal failure, nephrotic syndrome; those receiving immunosuppressive chemotherapy (including corticosteroids); and those who have received an organ or bone marrow transplant, and residents of nursing homes or long-term care facilities.
CDC recommends a yearly seasonal flu vaccine as the first and most important step in protecting against seasonal flu
- Annual flu vaccination is especially important for people at high risk of serious flu complications, including young children, pregnant women, people with certain chronic health conditions like asthma, diabetes or heart and lung disease, neurologic conditions and older adults.
- Seasonal flu vaccine also is important for healthcare workers and other people who live with or care for high risk people to prevent giving the flu to those at high risk.
- A seasonal vaccine will not protect you against 2009 H1N1 flu.
A new vaccine against 2009 H1N1 flu is available and is our best option for prevention of 2009 H1N1 flu infection.
- People included in the target groups to receive the first available doses of vaccine include children and young adults age 6 months -24 years, pregnant women, and people age 25-64 with chronic health conditions, household and close contacts of infants less than 6 months of age (since infants cannot be vaccinated) and healthcare and emergency medical services personnel.
Could the cases of invasive pneumococcal disease in Colorado have been prevented with pneumococcal vaccines?
Yes, some of them. Overall, 75% of Colorado’s invasive pneumococcal disease (IPD) cases were caused by pneumococcal serotypes that are in the 23-valent pneumococcal polysaccharide vaccine (PPSV), which is consistent with what we see during non-pandemic years. The majority of the cases (about two-thirds) had at least one indication to receive PPSV, but had not actually been vaccinated. Of the 10 IPD cases with documented 2009 H1N1 flu infection, 7 had conditions known to increase the risk of pneumococcal disease but only 1 had received PPSV. Five of 7 cases were caused by serotypes included in PPSV.
What percentage of adults with high risk conditions has received the pneumococcal polysaccharide vaccine?
Vaccination coverage for adults with high risk conditions recommended to receive the pneumococcal polysaccharide vaccine (PPSV) is low. Of adults 19 through 64 years of age with high risk conditions, 24.9% had received PPSV by the time they were interviewed in 2008. More details on PPSV vaccination coverage estimates in the United States for 2008 are available from the National Health Interview Survey (NHIS).How is invasive pneumococcal disease diagnosed in adults?
Most adults with invasive pneumococcal disease have pneumococcal pneumonia. Existing guidelines recommend the use of a commercially available urine antigen test (Binax NOW®) for the diagnosis of pneumococcal pneumonia in adults. Such testing, along with blood cultures and testing for flu infection, can assist clinicians in determining whether secondary pneumococcal pneumonia is occurring.
How is invasive pneumococcal disease treated in adults during flu season?
In communities where flu is circulating, treatment with flu antiviral agents is recommended for all hospitalized patients with confirmed, probable or suspected 2009 H1N1 or seasonal flu and for outpatients who are at higher risk for flu-related complications. Empiric treatment of patients hospitalized with invasive pneumococcal disease should include both flu antiviral agents and appropriate antibiotic therapy. For more information on treatment of influenza, see "Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season".
Where can I find more information about invasive pneumococcal disease, pneumococcal vaccines, and flu vaccines?
- Questions and Answers: Prevention Of Pneumococcal Infections Secondary To Seasonal And 2009 H1N1 Influenza
- Pneumococcal Vaccine Website
- For Clinicians: Prevention Of Pneumococcal Infections Secondary To Seasonal And 2009 H1N1 Influenza Viruses Infection
- Interim guidance for use of 23-valent pneumococcal polysaccharide vaccine during novel influenza A (H1N1) outbreak
- Preventing Seasonal Flu With Vaccination
- General Information About 2009 H1N1 Vaccines
- Links to non-federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the federal government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.
Get email updates
To receive weekly email updates about this site, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - Contact CDC-INFO

