2010 Guidelines
2010 Guidelines for the Prevention of Perinatal Group B Streptococcal Disease
HTML | PDF [1.54 MB, 36 pages]
Revised Guidelines for the Prevention of Perinatal Group B Streptococcal (GBS) Disease were published in the Morbidity and Mortality Weekly Report (MMWR) on November 19, 2010. These 2010 guidelines were developed using an evidence-based approach in collaboration with several professional associations. They received formal endorsements from:
- American Academy of Family Physicians (AAFP)
- American Academy of Pediatrics (AAP)
- American College of Nurse-Midwives (ACNM)
- American College of Obstetricians and Gynecologists (ACOG)
- American Society for Microbiology (ASM)
Background
Despite excellent progress in reducing the burden of GBS disease in the first week of life, commonly referred to as early-onset disease, GBS continues to be the leading cause of early-onset sepsis and meningitis in the United States. The primary risk factor for early-onset GBS disease is maternal colonization with GBS. About 10-30% of pregnant women are colonized. Intrapartum antibiotic prophylaxis (IAP) is highly effective at preventing early-onset GBS disease among infants born to colonized women.
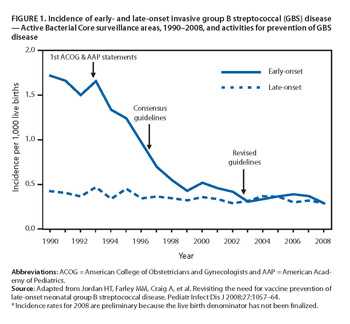
FIGURE 1. Incidence of early- and late-onset invasive group B streptococcal (GBS) disease [1 page]
Image reference: Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of Universal Antenatal Screening for Group B Streptococcus . N Eng J Med. 2009;360(25):2626-36.
CDC first issued guidelines on use of IAP for prevention of GBS disease in 1996. Those guidelines were revised in 2002, when it was recommended that all women undergo vaginal-rectal screening for GBS colonization at 35-37 weeks’ gestation to identify which women should receive IAP. Implementation of the 2002 guidelines has been quite good. One study found that 85% of pregnant women were screened for GBS colonization. Among those screened, 98% had results available at time of delivery. Eighty-five percent of women with an indication for IAP were administered treatment.
Nonetheless, there is room for improvement. Despite a recommendation that all women delivering preterm with unknown GBS status should be screened for GBS on admission and receive IAP, only 18% of those women were screened, and less than two thirds received IAP.
Implementation was also suboptimal for penicillin-allergic women. Penicillin-allergic women with an indication for IAP should receive cefazolin unless they are at high risk for anaphylaxis. However, 70% of women at low risk for anaphylaxis who received IAP were given clindamycin. This drug is not established as effective at preventing early-onset GBS disease, does not reach the fetal circulation or amniotic fluid well, and an increasing proportion of GBS isolates are resistant to it.
By successfully implementing the new 2010 guidelines, even more cases of early-onset GBS disease can be prevented.
Top of Page- Page last reviewed: May 23, 2016
- Page last updated: June 1, 2014
- Content source:



 ShareCompartir
ShareCompartir
