Ethylene Glycol and Propylene Glycol Toxicity
What Is the Biological Fate of Ethylene Glycol?
Course: WB 1103
CE Original Date: October 3, 2007
CE Renewal Date: October 3, 2010
CE Expiration Date: October 3, 2012
Download Printer-Friendly version [PDF - 496 KB]
| Previous Section | Next Section |
Learning Objectives |
Upon completion of this section, you should be able to
|
Introduction |
Ethylene glycol is rapidly absorbed from the gastrointestinal tract and slowly absorbed through the skin or lungs. The toxicity of ethylene glycol results from its metabolism to more toxic metabolites. Like ethanol, ethylene glycol is rapidly absorbed in the GI tract, with peak absorption in 30-60 minutes. |
Rapid Transformation |
Because it is highly water-soluble, ethylene glycol is distributed throughout total body water. Peak tissue levels occur several hours after ingestion. Approximately 24 to 48 hours later, it is difficult to detect ethylene glycol in urine or tissues, thus indicating rapid biotransformation. The normal serum half-life of ethylene glycol has been estimated to be about 2.5 hours in children and 3-8 hours in untreated adults. |
Metabolism in the Liver |
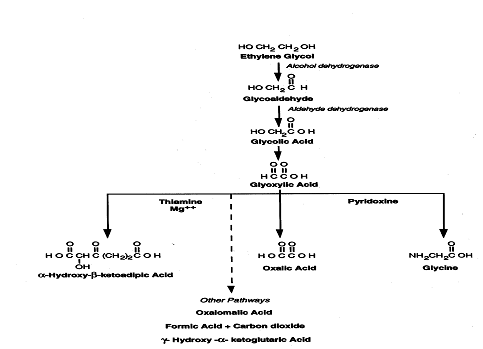
Other than its inebriating effects, ethylene glycol has relatively low toxicity. However, ethylene glycol is metabolized in the liver by successive oxidations to a variety of compounds that include
These compounds are more toxic than ethylene glycol itself (Figure 1) (Jacobsen and McMartin 1986; Hall AH 1992; Goldfrank LR 1998). Some of these compounds have elimination half-lives of up to 12 hours.
|
The Role of Alcohol Dehydrogenase |
The rate-limiting step in this metabolic process is the conversion of ethylene glycol to glycoaldehyde, a process that is catalyzed by alcohol dehydrogenase (ADH). Several factors may influence susceptibility to ethylene glycol-induced toxicity, including
Concomitant or recent ethanol exposure can decrease or prevent toxicity by preferentially competing for ADH, thereby inhibiting transformation of ethylene glycol to glycoaldehyde. Coadministration of ethanol increases the percentage of ethylene glycol excreted unchanged in the urine. Under normal conditions only a small fraction of ethylene glycol (less than 20% after low-dose ingestion) is excreted unchanged. |
Fomepizole |
A new medication, fomepizole or 4-methylpyrazole, targets the ADH enzyme as well (Baud, Galliot et al. 1988; Brent, McMartin et al. 1999; Jones and Volans 1999). Both ethanol and fomepizole are used therapeutically to treat ethylene glycol intoxication. Ethanol increases the half-life of ethylene glycol in the body to 17-18 hours; fomepizole increases the half-life to 11-14.75 hours. |
Key Points |
|
Progress Check |
| Previous Section | Next Section |