Facts About the Laboratory Response Network
In 1999, the Centers for Disease Control and Prevention (CDC) established the Laboratory Response Network (LRN). The LRN’s purpose is to run a network of labs that can respond to biological and chemical threats, and other public health emergencies. The LRN has grown since its inception. It now includes state and local public health, veterinary, military, and international labs. This fact sheet provides a brief description of the LRN, and how it works.
The LRN Mission
The LRN and its partners will maintain an integrated national and international network of laboratories that are fully equipped to respond quickly to acts of chemical or biological threats, emerging infectious diseases, and other public health threats and emergencies.
What is the LRN?
The LRN is a national network of more than 150 labs. The network includes the following types of labs:
- Federal—These include labs at CDC, the US Department of Agriculture, the Food and Drug Administration (FDA), and other facilities run by federal agencies.
- State and local public health—These are labs run by state and local departments of health. In addition to being able to test for Category A biological agents, a few LRN public health labs are able to measure human exposure to toxic chemicals through tests on clinical specimens.
- Military—Labs operated by the Department of Defense located both within the United States and abroad.
- Food testing—The LRN includes FDA and USDA labs, and others that are responsible for ensuring the safety of the food supply.
- Environmental—Includes labs that that are capable of testing water and other environmental samples.
- Veterinary—Some LRN labs, such as those run by USDA, are responsible for animal testing. Some diseases can be shared by humans and animals, and animals often provide the first sign of disease outbreak.
- International—The LRN has labs located in Canada, the United Kingdom, Australia, Mexico, and South Korea.
The LRN in Action
Anthrax attacks of 2001
The LRN has been put to the test on several occasions. In 2001, a Florida LRN reference laboratory discovered the presence of Bacillus anthracis in a clinical specimen. B. anthracis causes anthrax. LRN labs tested 125,000 samples by the time the investigation was completed. This amounted to more than 1 million separate tests.
Avian Influenza
The LRN played a vital role in the development and deployment of a new test that can detect the H5N1 virus. CDC staff responsible for coordinating LRN activities, along with other agency subject matter experts, developed a rapid detection test. The new test is also FDA approved as an in vitro diagnostic test. FDA approved the test and reagent kit in February 2006.
BioWatch
BioWatch is a program using air samplers to test for threat agents. The samplers are located in undisclosed cities and monitor the air 24 hours a day, 7 days a week. LRN BioWatch labs test filters from these samplers. Tests include polymerase chain reaction (PCR). PCR can quickly detect the presence of an agent’s unique DNA.
Severe Acute Respiratory Syndrome
CDC labs identified the unique DNA sequence of the virus that causes severe acute respiratory syndrome (SARS). The LRN developed tests and materials needed to support these tests. LRN gave member labs access to the tests and materials.
The LRN Structure for Biological Threats
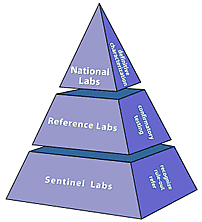
LRN labs are designated as either national, reference, or sentinel. Designation depends on the types of tests a laboratory can perform and how it handles infectious agents to protect workers and the public.
National labs have unique resources to handle highly infectious agents and the ability to identify specific agent strains.
Reference labs, can perform tests to detect and confirm the presence of a threat agent. These labs ensure a timely local response in the event of a threat incident. Rather than having to rely on confirmation from labs at CDC, many reference labs are capable of producing conclusive results. This allows local authorities to respond quickly to emergencies.
Sentinel labs represent the thousands of hospital-based labs that are on the front lines. Sentinel labs have direct contact with patients. Sentinel labs could be the first facility to spot a suspicious specimen. A sentinel laboratory’s responsibility is to refer a suspicious sample to the right reference lab.
LRN structure for Chemical Threats
CDC funding supports 62 U.S. states, territories, and metropolitan areas through the Public Health Emergency Preparedness cooperative agreement. In 2010, fifty-three laboratories within these jurisdictions provide emergency response capabilities for their local areas, the nation, or both. These laboratories make up the chemical component of the Laboratory Response Network (LRN).
A designation of Level 1, 2, or 3 identifies laboratory capabilities and defines member network participation. (Please note that the level designations changed in early 2005 so that laboratories previously designated “Level 1” are now “Level 3,” and laboratories previously designated “Level 3” are now “Level 1.”)
Level 3 Laboratories
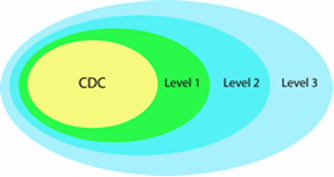
Nine laboratories are designated as Level 3 laboratories. All 53 laboratories have Level 3 capacity. These laboratories work with hospitals and other first responders within their jurisdiction to maintain competency in clinical specimen collection, storage, and shipment.
Level 2 Laboratories
Thirty-four labs are designated as Level 2 laboratories. Chemists in these laboratories are trained to detect exposure to a number of toxic chemical agents. Analysis of cyanide, nerve agents, and toxic metals in human samples are examples of Level 2 activities.
Level 1 Laboratories
Ten laboratories currently participate in Level 1 activities. These laboratories, which serve as surge-capacity laboratories for CDC, are able to detect not only the toxic chemical agents that Level 2 laboratories can detect but also can detect exposure to an expanded number of chemicals, including mustard agents, nerve agents, and other toxic industrial chemicals. Using unique high-throughput analysis capabilities, they expand CDC’s ability to analyze large number of patient samples when responding to large-scale exposure incidents.
How Do Public Health Labs Become LRN Members?
State lab directors determine whether public health labs in their states should be included in the network. Membership is not automatic. Prospective reference labs must have the equipment, trained personnel, properly designed facilities, and must demonstrate testing accuracy. State lab directors determine the criteria for inviting sentinel labs to join the LRN.
Partnerships
The LRN is also a partnership between government and private organizations that have a stake in biological threats and chemical preparedness. CDC runs the program with direction and recommendations provided by the following agencies and organizations:
- The Association of Public Health Laboratories;
- The Federal Bureau of Investigation (Department of Justice);
- The American Association of Veterinary Laboratory Diagnosticians;
- The American Society for Microbiology;
- The Environmental Protection Agency;
- The US Department of Agriculture;
- The Department of Defense;
- The US Food and Drug Administration;
- The Department of Homeland Security.
- Page last reviewed: May 2, 2013
- Page last updated: September 30, 2014
- Content source:
- Maintained By:





 ShareCompartir
ShareCompartir