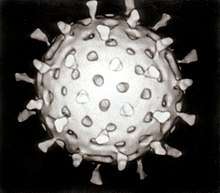
Virophage
Virophages are small, double stranded DNA viral phages that require the co-infection of another virus. The co-infecting viruses are typically giant viruses. Virophages rely on the viral replication factory of the co-infecting giant virus for their own replication. One of the characteristics of virophages is that they have a parasitic relationship with the co-infecting virus. Their dependence upon the giant virus for replication often results in the deactivation of the giant viruses. The virophage may improve the recovery and survival of the host organism.
| Lavidaviridae | |
|---|---|
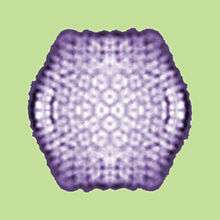 | |
| Sputnik virophage | |
| Virus classification | |
| (unranked): | Virus |
| Phylum: | incertae sedis |
| Class: | incertae sedis |
| Order: | incertae sedis |
| Family: | Lavidaviridae |
| Genera and Species | |
| |
Discovery
The first virophage was discovered in a cooling tower in Paris, France. It was discovered with its co-infecting giant virus, Acanthamoeba castellanii mamavirus (ACMV). The virophage was named Sputnik and its replication relied entirely on the co-infection of ACMV and its cytoplasmic replication machinery. Sputnik was also discovered to have an inhibitory effect on ACMV and improved the survival of the host. The most current list of discovered virophages include Sputnik, Sputnik 2, Sputnik 3, Zamilon and Mavirus[1][2]
A majority of these virophages are being discovered by analyzing metagenomic data sets. In metagenomic analysis, DNA data is sequenced and ran through multiple bioinformatic algorithms which, based on what is being analyzed, pull out certain important patterns and characteristics. In these data sets are giant viruses and virophages. They are separated by looking for sequences around 17 to 20 kbp (kilobasepairs) long and have similarities to already sequenced virophages. These virophages can have linear or circular double stranded DNA genomes.[3] Virophages in culture have icosahedral capsid particles that measure around 40 to 80 nanometers long.[4] Virophage particles are so small that electron microscopy must be used to view these particles. Metagenomic sequence-based analyses have been used to predict around 57 complete and partial virophage genomes [5] and most recently to identify 328 high-quality (complete or near-complete) genomes from diverse habitats including the human gut, plant rhizosphere, and terrestrial subsurface, from 27 distinct taxonomic clades [6].
Host Range and Replication
Virophages need to have a co-infecting virus in order for them to replicate. The virophages do not have the necessary enzymes to replicate on their own. Virophages use the giant viral replication machinery to replicate their own genomes and continue their existence. The host range for virophages include giant viruses with double stranded DNA genomes. Virophages use the transcriptional machinery of these giant viruses for their own replication instead of the host's transcriptional machinery. For example, the discovery of the virophage associated with the Samba virus decreased the viruses concentration in the host while the virophage was replicating using the giant virus. The host amoeba also showed a partial recovery from the infection by the Samba virus.[3]
Genome
Virophages have small double stranded DNA genomes that are either circular or linear in shape. The size of these genomes can vary depending on the giant virus it infects. Most virophages have genomes around 17–30 kbp (kilobasepairs).[4][5] Their genome is protected by an icosahedral capsid measuring approximately 40–80 nm in length.[4] In contrast, their co-infecting giant virus counterparts can have genomes as large as 1–2 Mbp (megabasepairs).[3] Some of the largest genomes of virophages are similar to the genome size of an adenovirus.[4]
| Genome
Size (kbp) |
Particle Size
(diameter, in nm) | |
|---|---|---|
| Virus: Poliovirus | 7 | 30 |
| Virus: Adenoviridae | 26–48 | 90–100 |
| Virophage: Zamilon Virophage | 17 | 50–60 |
| Virophage: Sputnik Virophage | 18 | 74 |
| Giant Virus: Cafeteria roenbergensis virus | 700 | 75 |
| Giant Virus: Mimivirus | 1,181 | 400–800 |
Distinguishable Characteristics
Unlike satellite viruses, virophages have a parasitic effect on their co-infecting virus. Virophages have been observed to render a giant virus inactive and thereby improve the condition of the host organism.
Taxonomy
The family Lavidaviridae with the two genera, Sputnikvirus and Mavirus, has been established by the International Committee on Taxonomy of Viruses for classification of virophages.[4]
- Family Lavidaviridae
- Genus Sputnikvirus
- Species Mimivirus-dependent virus Sputnik
- Species Mimivirus-dependent virus Zamilon
- Genus Mavirus
- Species Cafeteriavirus-dependent mavirus
- Genus Sputnikvirus
- Unassigned genus
- Organic Lake virophage
Additionally, virophage genomes identified from metagenomes have been classified together with the isolate virophages into 27 distinct clades with consistent genome length, gene content, and habitat distribution [6]
In popular culture
- The Radiolab podcast Shrink produced by National Public Radio featured journalist Carl Zimmer discussing giant viruses and virophages
References
- Fischer MG, Suttle CA (April 2011). "A virophage at the origin of large DNA transposons". Science. 332 (6026): 231–4. Bibcode:2011Sci...332..231F. doi:10.1126/science.1199412. PMID 21385722.
- Fischer MG, Hackl (December 2016). "Host genome integration and giant virus-induced reactivation of the virophage mavirus". Nature. 540 (7632): 288–91. Bibcode:2016Natur.540..288F. doi:10.1038/nature20593. PMID 27929021.
- Katzourakis, Aris; Aswad, Amr (2014). "The origins of giant viruses, virophages and their relatives in host genomes". BMC Biology. 12: 2–3. doi:10.1186/s12915-014-0051-y. PMC 4096385. PMID 25184667.
- Krupovic, Mart; Kuhn, Jens; Fischer, Metthias (Fall 2015). "A classification system for virophages and satellite viruses" (PDF). Archives of Virology. 161 (1): 233–247. doi:10.1007/s00705-015-2622-9. PMID 26446887 – via Springer.
- Roux, Simon; Chan, Leong-Keat; Egan, Rob; Malmstrom, Rex R.; McMahon, Katherine D.; Sullivan, Matthew B. (2017-10-11). "Ecogenomics of virophages and their giant virus hosts assessed through time series metagenomics". Nature Communications. 8 (1): 858. Bibcode:2017NatCo...8..858R. doi:10.1038/s41467-017-01086-2. ISSN 2041-1723. PMC 5636890. PMID 29021524.
- Paez-Espino, David; Zhou, Jinglie; Roux, Simon; Nayfach, Stephen; Pavlopoulos, Georgios A.; Schulz, Frederik; McMahon, Katherine D.; Walsh, David; Woyke, Tanja; Ivanova, Natalia N.; Eloe-Fadrosh, Emiley A.; Tringe, Susannah G.; Kyrpides, Nikos C. (2019-12-10). "Diversity, evolution, and classification of virophages uncovered through global metagenomics". Microbiome. 7 (1): 157. doi:10.1186/s40168-019-0768-5. PMID 31823797.