Transurethral microwave thermotherapy
Transurethral microwave thermotherapy (TUMT) is one of a number of effective and safe[1] procedures used in the treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia. It is an alternative treatment to pharmacotherapy such as alpha blockers, transurethral resection of the prostate (TURP), transurethral needle ablation of the prostate, photoselective vaporization of the prostate and prostatic removal or prostatectomy.[2]
| Transurethral microwave thermotherapy (TUMT) | |
|---|---|
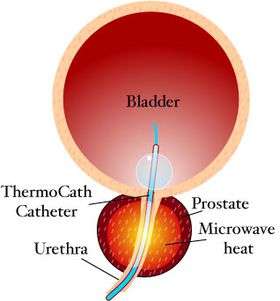 Transurethral microwave thermotherapy catheter in situ | |
| ICD-9-CM | 60.96 |
| MeSH | D020728 |
Process
Transurethral microwave thermotherapy is a non-surgical, minimally invasive therapy that can be performed under a local anesthetic on an outpatient basis. The treatment involves inserting a special microwave urinary catheter into the hyperplastic prostatic urethra. The microwave antenna within the catheter then emits microwaves to heat and destroy the surrounding prostatic tissue.
The procedure can take from 30 minutes to one hour and is well tolerated by patients. Following the procedure, the prostatic tissue will be swollen and irritated. Urologists often place a Foley catheter to prevent the patient from having urinary retention. After three to five days the Foley catheter can be replaced by a temporary prostatic stent to improve voiding without exacerbating irritation symptoms.[3]
Risks
The main risks of transurethral microwave thermotherapy include:
- Urinary retention
- Infection
- Postprocedural pain
- Retrograde ejaculation
- Intense pain during the procedure
- Urination pain for several weeks
Aftercare
The International Prostate Symptom Score including a quality of life survey, is often used to quantify symptoms and to monitor the response to the treatment. Convalescence is relatively rapid, with most patients able to void and a mean recovery time of less than 5 days at home.
However, prostatic edema is expected after microwave therapy, and this can lead to a risk of urinary retention. While some protocols suggest leaving a Foley catheter in for up to 2 weeks in all patients, other urologists are choosing to place a temporary prostatic stent after the first week following treatment. The stent is worn for 30 days and allows the patient to have volitional voiding with improved quality of life compared to a Foley catheter. Urinary flow generally improves over a few months.
Patients maintained on alpha-blockers after transurethral microwave thermotherapy may experience fewer urinary symptoms and have a decreased incidence of retention.[4]
Judgements in guidelines
- The American Urological Association (AUA) guidelines for the treatment of BPH from 2018 stated that TUMT may be offered to patients provided they are informed that it is associated with a higher risk of necessary retreatment compared to TURP.[5]
- The European Association of Urology (EAU) has - as of 2019 - removed TUMT from its guidelines.[6]
See also
References
- Stravodimos KG, Goldfischer ER, Klima WJ, Jabbour ME, Smith AD. 1998 Jun. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: a single-institution experience. | PMID 9609641
- Jonathan Rubenstein, MD | Transurethral Microwave Thermotherapy of the Prostate (TUMT) http://www.emedicine.com/med/topic3070.htm | Feb 6, 2008
- Dineen MK, Shore ND, Lumerman JH, Saslawsky MJ, Corica AP (2008 March 26). Use of a Temporary Prostatic Stent After Transurethral Microwave Thermotherapy Reduced Voiding Symptoms and Bother Without Exacerbating Irritative Symptoms. PMID 18374395
- Neal D. Shore; Martin K. Dineenb‡; Mark J. Saslawskyc; Jeffrey H. Lumermand; Alberto P. Corica (1999). "Prospective randomized comparison of high energy transurethral microwave thermotherapy versus alpha-blocker treatment of patients with benign prostatic hyperplasia". J. Urol. 161 (1): 139–43. doi:10.1016/S0022-5347(01)62084-6. PMID 10037386.
- Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS (2018). "Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline". J Urol. 200 (3): 612–619. doi:10.1016/j.juro.2018.05.048. PMID 29775639.CS1 maint: multiple names: authors list (link)
- EAU: Management of Non-neurogenic Male LUTS - Summary of Changes 2019.