Semen analysis
A semen analysis (plural: semen analyses), also called "seminogram"[1] evaluates certain characteristics of a male's semen and the sperm contained therein. It is done to help evaluate male fertility, whether for those seeking pregnancy or verifying the success of vasectomy. Depending on the measurement method, just a few characteristics may be evaluated (such as with a home kit) or many characteristics may be evaluated (generally by a diagnostic laboratory). Collection techniques and precise measurement method may influence results.
| Semen analysis | |
|---|---|
| Medical diagnostics | |
Human sperm stained for semen quality testing in the clinical laboratory. | |
| MedlinePlus | 003627 |
| HCPCS-L2 | G0027 |
Reasons for testing
The most common reasons for laboratory semen analysis in humans are as part of a couple's infertility investigation and after a vasectomy to verify that the procedure was successful. It is also commonly used for testing human donors for sperm donation, and for animals semen analysis is commonly used in stud farming and farm animal breeding.
Occasionally a man will have a semen analysis done as part of routine pre-pregnancy testing. At the laboratory level this is rare, as most healthcare providers will not test the semen and sperm unless specifically requested or there is a strong suspicion of a pathology in one of these areas discovered during the medical history or during the physical examination. Such testing is very expensive and time-consuming, and in the U.S. is unlikely to be covered by insurance. In other countries, such as Germany, the testing is covered by all insurances.
Relation to fertility
The characteristics measured by semen analysis are only some of the factors in semen quality. One source states that 30% of men with a normal semen analysis actually have abnormal sperm function.[2] Conversely, men with poor semen analysis results may go on to father children.[3] In NICE guidelines, mild male factor infertility is defined as when 2 or more semen analyses have 1 or more variables below the 5th percentile, and confers a chance of pregnancy occurring naturally through vaginal intercourse within 2 years similar to people with mild endometriosis.[4]
Collection methods
Different methods used for semen collection are masturbation, condom collection and epididymal extraction, etc. The sample should never be obtained through coitus interruptus for several reasons: Some part of ejaculation could be lost, bacterial contamination could happen and the acid vaginal pH could be detrimental for sperm motility. The optimal sexual abstinence for semen sample obtaining is from 2–7 days. The most common way to obtain a semen sample is through masturbation and the best place to obtain it is in the clinic where the analysis will take place in order to avoid temperature changes during the transport that can be lethal for some spermatozoa. Once the sample is obtained, it must be put directly in a sterile plastic recipient (never in a conventional preservative since they have chemical substances as lubricants or spermicides that could damage the sample) and be handed in the clinic for it to be studied within the following hour.
Parameters
Examples of parameters measured in a semen analysis are: sperm count, motility, morphology, volume, fructose level and pH.
Sperm count
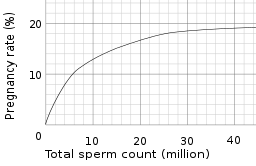
Sperm count, or sperm concentration to avoid confusion with total sperm count, measures the concentration of sperm in a man's ejaculate, distinguished from total sperm count, which is the sperm count multiplied with volume. Over 15 million sperm per milliliter is considered normal, according to the WHO in 2010.[5] Older definitions state 20 million.[2][3] A lower sperm count is considered oligozoospermia. A vasectomy is considered successful if the sample is azoospermic (zero sperm of any kind found). Some define success as when rare/occasional non-motile sperm are observed (fewer than 100,000 per millilitre).[6] Others advocate obtaining a second semen analysis to verify the counts are not increasing (as can happen with re-canalization) and others still may perform a repeat vasectomy for this situation.
Chips for home use are emerging that can give an accurate estimation of sperm count after three samples taken on different days. Such a chip may measure the concentration of sperm in a semen sample against a control liquid filled with polystyrene beads.[7]
Motility
The World Health Organization has a value of 50% and this must be measured within 60 minutes of collection. WHO also has a parameter of vitality, with a lower reference limit of 60% live spermatozoa.[5] A man can have a total number of sperm far over the limit of 20 million sperm cells per milliliter, but still have bad quality because too few of them are motile. However, if the sperm count is very high, then a low motility (for example, less than 60%) might not matter, because the fraction might still be more than 8 million per millilitre. The other way around, a man can have a sperm count far less than 20 million sperm cells per millilitre and still have good motility, if more than 60% of those observed sperm cells show good forward movement - which is beneficial because nature favours quality over quantity.
A more specified measure is motility grade, where the motility of sperm are divided into four different grades:[8]
- Grade a: Sperm with progressive motility. These are the strongest and swim fast in a straight line. Sometimes it is also denoted motility IV.
- Grade b: (non-linear motility): These also move forward but tend to travel in a curved or crooked motion. Sometimes also denoted motility III.
- Grade c: These have non-progressive motility because they do not move forward despite the fact that they move their tails. Sometimes also denoted motility II.
- Grade d: These are immotile and fail to move at all. Sometimes also denoted motility I.
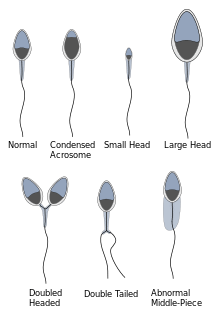
Morphology
Regarding sperm morphology, the WHO criteria as described in 2010 state that a sample is normal (samples from men whose partners had a pregnancy in the last 12 months) if 4% (or 5th centile) or more of the observed sperm have normal morphology.[5][9]
Morphology is a predictor of success in fertilizing oocytes during in vitro fertilization.
Up to 10% of all spermatozoa have observable defects and as such are disadvantaged in terms of fertilising an oocyte.[10]
Also, sperm cells with tail-tip swelling patterns generally have lower frequency of aneuploidy.[11]
A motile sperm organelle morphology examination (MSOME) is a particular morphologic investigation wherein an inverted light microscope equipped with high-power optics and enhanced by digital imaging is used to achieve a magnification above x6000, which is much higher than the magnification used habitually by embryologists in spermatozoa selection for intracytoplasmic sperm injection (x200 to x400).[12] A potential finding on MSOME is the presence of sperm vacuoles, which are associated with sperm chromatin immaturity, particularly in the case of large vacuoles.[13]
Volume
According to one lab test manual semen volumes between 2.0 mL and 5 mL are normal;[3] WHO regards 1.5 ml as the lower reference limit.[5] Low volume may indicate partial or complete blockage of the seminal vesicles, or that the man was born without seminal vesicles.[2] In clinical practice, a volume of less than 2 mL in the setting of infertility and absent sperm should prompt an evaluation for obstructive azoospermia. A caveat to this is be sure it has been at least 48 hours since the last ejaculation to time of sample collection.
The human ejaculate is mostly composed of water, 96 to 98% of semen is water. One way of ensuring that a man produces more ejaculate[14] is to drink more liquids. Men also produce more seminal fluid after lengthy sexual stimulation and arousal. Reducing the frequency of sex and masturbation helps increase semen volume. Sexually transmitted diseases also affect the production of semen. Men who are infected[15] with the human immunodeficiency virus (HIV) produce lower semen volume.
Color
Semen normally has a whitish-gray color. It tends to get a yellowish tint as a man ages. Semen color is also influenced by the food we eat: foods that are high in sulfur, such as garlic, may result in a man producing yellow semen.[16] Presence of blood in semen (hematospermia) leads to a brownish or red colored ejaculate. Hematospermia is a rare condition.
Semen that has a deep yellow color or is greenish in appearance may be due to medication. Brown semen is mainly a result of infection and inflammation of the prostate gland, urethra, epididymis and seminal vesicles. Other causes of unusual semen color include sexually transmitted infections such as gonorrhea and chlamydia, genital surgery and injury to the male sex organs.
Fructose level
Fructose level in the semen may be analysed to determine the amount of energy available to the semen for moving.[3] WHO specifies a normal level of 13 μmol per sample. Absence of fructose may indicate a problem with the seminal vesicles.[2]
pH
According to one lab test manual normal pH range is 7.1-8.0;[3] WHO criteria specify normal as 7.2-7.8.[2] Acidic ejaculate (lower pH value) may indicate one or both of the seminal vesicles are blocked. A basic ejaculate (higher pH value) may indicate an infection.[2] A pH value outside of the normal range is harmful to sperm and affect their ability to penetrate the egg.[3]
Liquefaction
The liquefaction is the process when the gel formed by proteins from the seminal vesicles is broken up and the semen becomes more liquid. It normally takes less than 20 minutes for the sample to change from a thick gel into a liquid. In the NICE guidelines, a liquefaction time within 60 minutes is regarded as within normal ranges.[17]
Viscosity
After liquefaction, the viscosity of the sample can be estimated by gently aspirating into a wide-bore (approximately 1,5 mm of diameter) plastic disposable pipette, allowing the semen to drop by gravity and observing the length of any thread. A normal sample leaves the pipette in small discrete drops. If viscosity is abnormal, the drop will form a thread more than 2 cm long. High viscosity can interfere with determination of sperm motility, sperm concentration, detection of antibody-coated spermatozoa and measurement of biochemical markers.[18]
MOT
MOT is a measure of how many million sperm cells per ml are highly motile,[19] that is, approximately of grade a (>25 micrometer per 5 sek. at room temperature) and grade b (>25 micrometer per 25 sek. at room temperature). Thus, it is a combination of sperm count and motility.
With a straw [20] or a vial volume of 0.5 milliliter, the general guideline is that, for intracervical insemination (ICI), straws or vials making a total of 20 million motile spermatozoa in total is recommended. This is equal to 8 straws or vials 0.5 ml with MOT5, or 2 straws or vials of MOT20. For intrauterine insemination (IUI), 1-2 MOT5 straws or vials is regarded sufficient.[21] In WHO terms, it is thus recommended to use approximately 20 million grade a+b sperm in ICI, and 2 million grade a+b in IUI.
DNA damage
DNA damage in sperm cells that is related to infertility can be probed by analysis of DNA susceptibility to denaturation in response to heat or acid treatment [22] and/or by detection of DNA fragmentation revealed by the presence of double-strand breaks detected by the TUNEL assay.[23][24]
Total motile spermatozoa
Total motile spermatozoa (TMS)[25] or total motile sperm count (TMSC)[26] is a combination of sperm count, motility and volume, measuring how many million sperm cells in an entire ejaculate are motile.
Use of approximately 20 million sperm of motility grade c or d in ICI, and 5 million ones in IUI may be an approximate recommendation.
Others
The NICE guidelines also include testing vitality, with normal ranges defined as more than 75% of sperm cells alive.[17]
The sample may also be tested for white blood cells. A high level of white blood cells in semen is called leucospermia and may indicate an infection.[2] Cutoffs may vary, but an example cutoff is over 1 million white blood cells per milliliter of semen.[2]
Abnormalities
- Aspermia: absence of semen
- Azoospermia: absence of sperm
- Hypospermia: low semen volume
- Hyperspermia: high semen volume
- Oligozoospermia: Very low sperm count
- Asthenozoospermia: poor sperm motility
- Teratozoospermia: sperm carry more morphological defects than usual
- Necrozoospermia: all sperm in the ejaculate are dead
- Leucospermia: a high level of white blood cells in semens
Factors that influence results
Apart from the semen quality itself, there are various methodological factors that may influence the results, giving rise to inter-method variation.
Compared to samples obtained from masturbation, semen samples from collection condoms have higher total sperm counts, sperm motility, and percentage of sperm with normal morphology . For this reason, they are believed to give more accurate results when used for semen analysis.
If the results from a man's first sample are subfertile, they must be verified with at least two more analyses. At least 2 to 4 weeks must be allowed between each analysis.[27] Results for a single man may have a large amount of natural variation over time, meaning a single sample may not be representative of a man's average semen characteristics. In addition, sperm physiologist Joanna Ellington believes that the stress of producing an ejaculate sample for examination, often in an unfamiliar setting and without any lubrication (most lubricants are somewhat harmful to sperm), may explain why men's first samples often show poor results while later samples show normal results.
A man may prefer to produce his sample at home rather than at the clinic. The site of semen collection does not affect the results of a semen analysis.[28]. If produced at home the sample should be kept as close to body temperature as possible as exposure to cold or warm conditions can effect sperm motility
Measurement methods
Volume can be determined by measuring the weight of the sample container, knowing the mass of the empty container. Sperm count and morphology can be calculated by microscopy. Sperm count can also be estimated by kits that measure the amount of a sperm-associated protein, and are suitable for home use.[29]
Computer assisted semen analysis (CASA) is a catch-all phrase for automatic or semi-automatic semen analysis techniques. Most systems are based on image analysis, but alternative methods exist such as tracking cell movement on a digitizing tablet.[30][31] Computer-assisted techniques are most-often used for the assessment of sperm concentration and mobility characteristics, such as velocity and linear velocity. Nowadays, there are CASA systems, based on image analysis and using new techniques, with near perfect results, and doing full analysis in a few seconds. With some techniques, sperm concentration and motility measurements are at least as reliable as current manual methods.[32]
Raman spectroscopy has made progress in its ability to perform characterization, identification and localization of sperm nuclear DNA damage.[33]
See also
- Semen quality
- Artificial insemination for more details of how semen parameters affects pregnancy rate
References
- Lozano GM, Bejarano I, Espino J, González D, Ortiz A, García JF, Rodríguez AB, Pariente JA (2009). "Density gradient capacitation is the most suitable method to improve fertilization and to reduce DNA fragmentation positive spermatozoa of infertile men". Anatolian Journal of Obstetrics & Gynecology. 3 (1): 1–7.
- "Understanding Semen Analysis". Stonybrook, State University of New York. 1999. Archived from the original on October 17, 2007. Retrieved 2007-08-05.
- RN, Kathleen Deska Pagana PhD; FACS, Timothy J. Pagana MD (2013-11-22). Mosby's Manual of Diagnostic and Laboratory Tests, 5e (5 ed.). St. Louis, Missouri: Mosby. ISBN 978-0-323-08949-4.
- Fertility: assessment and treatment for people with fertility problems. NICE clinical guideline CG156 - Issued: February 2013
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM (May–Jun 2010). "World Health Organization reference values for human semen characteristics" (PDF). Human Reproduction Update. 16 (3): 231–45. doi:10.1093/humupd/dmp048. PMID 19934213.
- Rajmil O, Fernández M, Rojas-Cruz C, Sevilla C, Musquera M, Ruiz-Castañe E (2007). "Azoospermia should not be given as the result of vasectomy". Arch. Esp. Urol. (in Spanish). 60 (1): 55–8. PMID 17408173.
Dhar NB, Bhatt A, Jones JS (2006). "Determining the success of vasectomy". BJU Int. 97 (4): 773–6. doi:10.1111/j.1464-410X.2006.06107.x. PMID 16536771. - New Chip Provides Cheap At-Home Sperm Counting By Stuart Fox Posted 01.26.2010 in Popular Science
- "Fertility Testing and Semen Analysis".
- Rothmann SA, Bort AM, Quigley J, Pillow R (2013). "Sperm morphology classification: a rational method for schemes adopted by the world health organization". Methods in Molecular Biology. 927: 27–37. doi:10.1007/978-1-62703-038-0_4. ISBN 978-1-62703-037-3. PMID 22992901.
- Sadler, T. (2010). Langman's medical embryology (11th ed.). Philadelphia: Lippincott William & Wilkins. p. 30. ISBN 978-0-7817-9069-7.
- Pang MG, You YA, Park YJ, Oh SA, Kim DS, Kim YJ (June 2009). "Numerical chromosome abnormalities are associated with sperm tail swelling patterns". Fertil. Steril. 94 (3): 1012–1020. doi:10.1016/j.fertnstert.2009.04.043. PMID 19505688.
- Oliveira JB, Massaro FC, Mauri AL, Petersen CG, Nicoletti AP, Baruffi RL, Franco JG (2009). "Motile sperm organelle morphology examination is stricter than Tygerberg criteria". Reproductive Biomedicine Online. 18 (3): 320–326. doi:10.1016/S1472-6483(10)60088-0. PMID 19298729.
- Perdrix A, Rives N (2013). "Motile sperm organelle morphology examination (MSOME) and sperm head vacuoles: State of the art in 2013". Human Reproduction Update. 19 (5): 527–541. doi:10.1093/humupd/dmt021. PMID 23825157.
- "How to increase your ejaculate". Retrieved 8 April 2017.
- "Semen alterations in HIV-1 infected men". Cite journal requires
|journal=(help) - "Causes of Yellow Semen and Yellow Sperm: What Color is Sperm?". 12 April 2016.
- "Archived copy" (PDF). Archived from the original (PDF) on 2010-08-03. Retrieved 2010-08-03.CS1 maint: archived copy as title (link) Fertility: Assessment and Treatment for People with Fertility Problems. London: RCOG Press. 2004. ISBN 978-1-900364-97-3.
- "WHO | WHO laboratory manual for the examination and processing of human semen". WHO. Retrieved 2019-12-08.
- "Frequently asked questions".
- "Frequently asked questions".
- "Frequently asked questions".
- Evenson DP, Darzynkiewicz Z, Melamed MR. (1980) Relation of mammalian sperm chromatin heterogeneity to fertility. Science 210:1131-1133; PMID 7444440
- Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. (1993) Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells. Analogy to apoptosis of somatic cells. Exp Cell Res 207:202-205; PMID 8391465
- Evenson DP. (2017) Evaluation of sperm chromatin structure and DNA strand breaks is an important part of clinical male fertility assessment. Transl Androl Urol. Sep;6(Suppl 4):S495-S500. doi 10.21037/tau.2017.07.20. PMID 29082168
- Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H (November 2008). "Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature". Fertil. Steril. 93 (1): 79–88. doi:10.1016/j.fertnstert.2008.09.058. PMID 18996517.
- Pasqualotto EB, Daitch JA, Hendin BN, Falcone T, Thomas AJ, Nelson DR, Agarwal A (October 1999). "Relationship of total motile sperm count and percentage motile sperm to successful pregnancy rates following intrauterine insemination" (PDF). J. Assist. Reprod. Genet. 16 (9): 476–82. doi:10.1023/A:1020598916080. PMC 3455631. PMID 10530401.
- Toni Weschler (2006). Taking Charge of Your Fertility (10th Anniversary ed.). New York: Collins. ISBN 0-06-088190-9.
- Licht RS, Handel L, Sigman M (2007). "Site of semen collection and its effect on semen analysis parameters". Fertil. Steril. 89 (2): 395–7. doi:10.1016/j.fertnstert.2007.02.033. PMID 17482174.
- dailyprogress.com > Charlottesville company sends out its home male sterility tests By Brian McNeill. Published: May 14, 2009
- Mortimer ST (1 July 2000). "CASA--practical aspects". J. Androl. 21 (4): 515–24. PMID 10901437. Retrieved 2007-08-05.
- Hinting A, Schoonjans F, Comhaire F (1988). "Validation of a single-step procedure for the objective assessment of sperm motility characteristics". Int. J. Androl. 11 (4): 277–87. doi:10.1111/j.1365-2605.1988.tb01001.x. PMID 3170018.
- Testing of Accubead in: Tomlinson MJ, Pooley K, Simpson T, Newton T, Hopkisson J, Jayaprakasan K, Jayaprakasan R, Naeem A, Pridmore T (April 2010). "Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms". Fertil. Steril. 93 (6): 1911–20. doi:10.1016/j.fertnstert.2008.12.064. PMID 19200972.
- Mallidis C, Sanchez V, Wistuba J, Wuebbeling F, Burger M, Fallnich C, Schlatt S (2014). "Raman microspectroscopy: shining a new light on reproductive medicine". Hum. Reprod. Update. 20 (3): 403–14. doi:10.1093/humupd/dmt055. PMID 24144514.
External links
- Blood in semen Hematospermia
- Semen analysis - Lab Tests Online
- Geneva Foundation for Medical Education and Research - complete list of parameters.
- WHO laboratory manual for the examination and processing of human semen Fifth edition 2010 - updated, standardized, evidence-based procedures and recommendations for laboratory analysis and quality control