Sapanisertib
Sapanisertib (also known as INK128 and TAK-228) is an experimental small molecule inhibitor of mTOR which is administered orally. It targets both mTORC1 and mTORC2.[1]
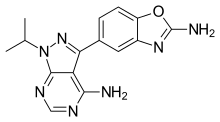 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H15N7O |
| Molar mass | 309.333 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Developed by Millennium Pharmaceuticals,[2] sapanisertib is in phase II clinical trials for breast cancer, endometrial cancer, glioblastoma, renal cell carcinoma, and thyroid cancer.[3] The drug has been well tolerated by patients with advanced solid tumours in Phase I trials.[4]
References
- "sapanisertib". NCI Drug Dictionary. National Cancer Institute. 2 February 2011. Retrieved 23 August 2019.
- https://searchusan.ama-assn.org/usan/documentDownload?uri=%2Funstructured%2Fbinary%2Fusan%2Fsapanisertib.pdf
- http://adisinsight.springer.com/drugs/800030541
- Moore, Kathleen N; Bauer, Todd M; Falchook, Gerald S; Chowdhury, Swapan; Patel, Chirag; Neuwirth, Rachel; Enke, Aaron; Zohren, Fabian; Patel, Manish R (February 2018). "Phase I study of the investigational oral mTORC1/2 inhibitor sapanisertib (TAK-228): tolerability and food effects of a milled formulation in patients with advanced solid tumours". ESMO Open. 3 (2): e000291. doi:10.1136/esmoopen-2017-000291.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.