Progenitor cell
A progenitor cell is a biological cell that, like a stem cell, has a tendency to differentiate into a specific type of cell, but is already more specific than a stem cell and is pushed to differentiate into its "target" cell. The most important difference between stem cells and progenitor cells is that stem cells can replicate indefinitely, whereas progenitor cells can divide only a limited number of times. Controversy about the exact definition remains and the concept is still evolving.[1]
The terms "progenitor cell" and "stem cell" are sometimes equated.[2]
Properties
Most progenitors are described as oligopotent. In this point of view, they may be compared to adult stem cells. But progenitors are said to be in a further stage of cell differentiation. They are in the “center” between stem cells and fully differentiated cells. The kind of potency they have depends on the type of their "parent" stem cell and also on their niche. Some progenitor cells were found during research, and were isolated. After their marker was found, it was proven that these progenitor cells could move through the body and migrate towards the tissue where they are needed. Many properties are shared by adult stem cells and progenitor cells.
Progenitor cells are found in adult organisms and they act as a repair system for the body. They replenish special cells, but also maintain the blood, skin and intestinal tissues. They can also be found in developing embryonic pancreatic tissue.
| Stem Cell | Progenitor Cell | |
|---|---|---|
| Self-renewal in vitro | Unlimited | Limited |
| Potentiality | Multipotent | Unipotent, sometimes oligopotent |
| Maintenance of self-renewal | Yes | No |
| Population | Reaches maximum number of cells before differentiating | Does not reach maximum population |
Function
The majority of progenitor cells lie dormant or possess little activity in the tissue in which they reside. They exhibit slow growth and their main role is to replace cells lost by normal attrition. In case of tissue injury, damaged or dead cells, progenitor cells can be activated. Growth factors or cytokines are two substances that trigger the progenitors to mobilize toward the damaged tissue. At the same time, they start to differentiate into the target cells. Not all progenitors are mobile and are situated near the tissue of their target differentiation. When the cytokines, growth factors and other cell division-enhancing stimulators take on the progenitors, a higher rate of cell division is introduced. It leads to the recovery of the tissue.
Examples
The characterization or the defining principle of progenitor cells, in order to separate them from others, is based on the different cell markers rather than their morphological appearance.[3]
- Satellite cells found in muscles. They play a major role in muscle cell differentiation and injury recoveries.
- Intermediate progenitor cells formed in the subventricular zone.[4] Some of these transit amplifying neural progenitors migrate via rostral migratory stream to the olfactory bulb and differentiate further into specific types of neural cells.
- Radial glial cells found in developing regions of the brain, most notably the cortex. These progenitor cells are easily identified by their long radial process.
- Bone marrow stromal cells found in the epidermis and make up 10% of progenitor cells. They are often classed as stem cells due to their high plasticity and potential for unlimited capacity for self-renewal.
- Periosteum contains progenitor cells that develop into osteoblasts and chondroblasts.
- Pancreatic progenitor cells are among the most studied progenitors.[5] They are used in research to develop a cure against diabetes type-1.
- Angioblasts or endothelial progenitor cells (EPC). These are very important for research on fracture and wounds healing.[6]
- Blast cells are involved in generation of B- and T-lymphocytes, which participate in immune responses.[7][8]
- Boundary cap cells from the neural crest form a barrier between the cells of the central nervous system and cells of the peripheral nervous system.[9]
Development of the human cerebral cortices
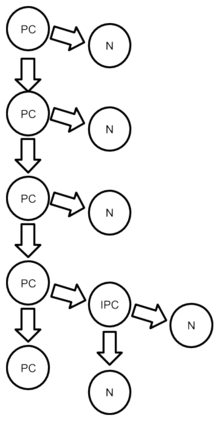
Before embryonic day 40 (E40), progenitor cells generate other progenitor cells; after that period, progenitor cells produce only dissimilar mesenchymal stem cell daughters. The cells from a single progenitor cell form a proliferative unit that creates one cortical column; these columns contain a variety of neurons with different shapes.[10]
See also
- Induced progenitor stem cells
- Endothelial progenitor cell
References
- Seaberg, R. M.; Van Der Kooy, D. (2003). "Stem and progenitor cells: The premature desertion of rigorous definitions". Trends in Neurosciences. 26 (3): 125–131. doi:10.1016/S0166-2236(03)00031-6. PMID 12591214.
- "progenitor cell" at Dorland's Medical Dictionary
- Morgan, JE; Partridge, TA (August 2003). "Muscle satellite cells". The International Journal of Biochemistry & Cell Biology. 35 (8): 1151–6. doi:10.1016/s1357-2725(03)00042-6. PMID 12757751.
- Noctor SC, Martínez-Cerdeño V, Kriegstein AR (May 2007). "Contribution of intermediate progenitor cells to cortical histogenesis". Arch. Neurol. 64 (5): 639–42. doi:10.1001/archneur.64.5.639. PMID 17502462.
- Awong, G. (2011). "Thymus-bound: The many features of T cell progenitors". Frontiers in Bioscience: 961. doi:10.2741/200.
- Barber, C. L.; Iruela-Arispe, M. L. (2006). "The Ever-Elusive Endothelial Progenitor Cell: Identities, Functions and Clinical Implications". Pediatric Research. 59 (4 Pt 2): 26R–32R. doi:10.1203/01.pdr.0000203553.46471.18. PMID 16549545.
- Carotta, S.; Nutt, S. L. (2008). "Losing B cell identity". BioEssays. 30 (3): 203–207. doi:10.1002/bies.20725. PMID 18293359.
- Awong, G. (2011). "Thymus-bound: The many features of T cell progenitors". Frontiers in Bioscience: 961. doi:10.2741/200.
- Monk, KR; Feltri, ML; Taveggia, C (August 2015). "New insights on Schwann cell development". Glia. 63 (8): 1376–93. doi:10.1002/glia.22852. PMC 4470834. PMID 25921593.
- Mason, John O.; Price, David J. (2016-10-15). "Building brains in a dish: Prospects for growing cerebral organoids from stem cells". Neuroscience. 334: 105–118. doi:10.1016/j.neuroscience.2016.07.048. ISSN 1873-7544. PMID 27506142.