Osmotic dilator
Osmotic dilators are medical implements used to dilate the uterine cervix by swelling as they absorb fluid from surrounding tissue.[1] They may be composed of natural or synthetic materials. A laminaria stick or tent is a thin rod made of the stems of dried Laminaria, a genus of kelp. Laminaria sticks can be generated from Laminaria japonica[2] and Laminaria digitata.[3] Synthetic osmotic dilators are commonly referred to by their brand names, such as Dilapan.[4] Dilapan-S are composed of polyacrylonitrile, a plastic polymer.[4] The hygroscopic nature of the polymer causes the dilator to absorb fluid and expand.
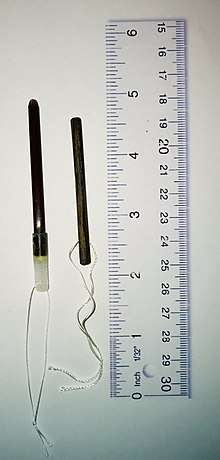
Use in obstetrics and gynecology
Osmotic dilators are most commonly used to slowly dilate and soften the cervix prior to surgical abortion, a process referred to as cervical preparation. Adequate cervical preparation is important prior to surgical abortions because it helps to prevent complications of dilation and evacuation (D&E), such as laceration of the cervix.[5] Cervical preparation can be accomplished with osmotic dilators, with medications such as prostaglandins and/or mifepristone, or with a combination of these. However, there is no consensus as to which cervical preparation method is superior in terms of safety and efficacy.[6]
At later gestational ages, osmotic dilators, including laminaria, may be used to assist in dilating the cervix. Most abortion providers use laminaria, Dilapan, or both for osmotic dilation prior to surgical abortion after 16–18 weeks gestation.[7]
Osmotic dilators may also be used to achieve cervical dilation prior to gynecology procedures, such as hysteroscopy or dilation and curettage of the non-pregnant uterus,[8] although this is uncommon.
Placement

Prior to a planned surgical abortion, osmotic dilator(s) may be inserted into a woman's cervix. A speculum is placed in the vagina to allow the provider to see the uterine cervix. A tenaculum may be placed on the anterior lip of the cervix to straighten the cervical canal and hold the cervix steady. Pain medications may be administered via a paracervical block. The dilator is then grasped with a ring forcep and is placed into the cervix so that it spans both the internal cervical os and external cervical os. Over time, the osmotic dilator absorbs fluid and swells to 3-4 times the initial diameter.[9] Most of the increase in size occurs within 6 hours after the dilator are placed in the cervix, though further expansion will continue over 12–24 hours.[7] The number of osmotic dilators placed depends on the degree of cervical dilation that is sought. This may be affected by the gestational age of the pregnancy and history of prior vaginal deliveries. More dilators are generally used with advancing gestational age.
Mechanism of action
Laminaria function by absorbing fluid from the surrounding tissue and expanding. Thus exerts radial pressure on the cervix. They also cause the release of prostaglandins.[11]
Removal
Laminaria are removed prior to initiating the D&E, after they have started the process of dilating the cervix. They are removed by grasping the strings of the dilator and applying gentle traction. The cervix may be dilated further using rigid cervical dilators which serially increase in diameter.[10]
Effectiveness
There is no evidence regarding the risk of not achieving vaginal delivery within 24 hours with laminaria compared to prostaglandin E2 but there may be a lower risk of uterine hyperstimulation with laminaria. It is not certain whether the risks of caesarian section, perinatal death or maternal death are reduced or increased with laminaria compared with vaginal or cervial prostaglandin E2.[12]
Risks
The incidence of complications related to osmotic[1] dilator use prior to abortion has not been systematically studied and reported, but serious complications appear to be very rare.[8] Risks of osmotic dilator insertion include pain,[13] rupture of amniotic membranes, initiation of labor, cervical or uterine perforation, retention of the dilator, and infection.[8][7]
Original Dilapan dilators were prone to fracturing under tension during removal which sometimes led to retention of dilator fragments within the uterus.[8] Dilapan was subsequently removed from the market and replaced with Dilapan-S which is less prone to fragmentation.[8] Dilapan may expand unevenly within the cervix and develop a dumbbell shape which can result in difficult removal.
References
- "Cervical (Osmotic) Dilator". MyHealth.Alberta.ca. Government of Alberta. Retrieved 6 August 2019.
- "Dilateria - Laminaria - for Cervical Dilation". Cooper Surgical. Retrieved 2019-07-09.
- Kazzi, G. M.; Bottoms, S. F.; Rosen, M. G. (1982). "Efficacy and safety of Laminaria digitata for preinduction ripening of the cervix". Obstetrics and Gynecology. 60 (4): 440–443. ISSN 0029-7844. PMID 7121931.
- "Discover a new approach in labor induction". Dilapan-S®. Retrieved 2019-08-27.
- "Second-Trimester Abortion - ACOG". www.acog.org. Retrieved 2019-07-09.
- Newmann, Sara J.; Dalve-Endres, Andrea; Diedrich, Justin T.; Steinauer, Jody E.; Meckstroth, Karen; Drey, Eleanor A. (2010-08-04). "Cervical preparation for second trimester dilation and evacuation". The Cochrane Database of Systematic Reviews (8): CD007310. doi:10.1002/14651858.CD007310.pub2. ISSN 1469-493X. PMID 20687085.
- Fox, Michelle C.; Krajewski, Colleen M. (February 2014). "Cervical preparation for second-trimester surgical abortion prior to 20 weeks' gestation". Contraception. 89 (2): 75–84. doi:10.1016/j.contraception.2013.11.001. PMID 24331860.
- Lichtenberg, E. Steve (2004). "Complications of osmotic dilators". Obstetrical & Gynecological Survey. 59 (7): 528–536. doi:10.1097/00006254-200407000-00022. PMID 15199271.
- "Laminaria (Cervical Dilator) by Medgyn Products, Inc". www.medline.com. Retrieved 2019-07-23.
- Lerma, Klaira; Blumenthal, Paul D. (2019-05-25). "Current and potential methods for second trimester abortion". Best Practice & Research Clinical Obstetrics & Gynaecology. doi:10.1016/j.bpobgyn.2019.05.006. ISSN 1521-6934. PMID 31281014.
- Gelber, Shari; Sciscione, Anthony (September 2006). "Mechanical methods of cervical ripening and labor induction". Clinical Obstetrics and Gynecology. 49 (3): 642–657. doi:10.1097/00003081-200609000-00022. ISSN 0009-9201. PMID 16885669.
- de Vaan, MDT; ten Eikelder, MLG; Jozwiak, M; Palmer, KR; Davies-Tuck, M; Bloemenkamp, KWM; Mol, BWJ; Boulvain, M (18 October 2019). "Mechanical methods for induction of labour". The Cochrane Database of Systematic Reviews. 10: CD001233. doi:10.1002/14651858.CD001233.pub3. PMC 6797496. PMID 31623014.
- Lerma, Klaira; Blumenthal, Paul D. (May 2019). "Current and potential methods for second trimester abortion". Best Practice & Research Clinical Obstetrics & Gynaecology. doi:10.1016/j.bpobgyn.2019.05.006. PMID 31281014.