Osilodrostat
Osilodrostat (INN, USAN) (developmental code name LCI-699) is an orally active, nonsteroidal corticosteroid biosynthesis inhibitor which is under development by Novartis for the treatment of Cushing's syndrome and pituitary ACTH hypersecretion (a specific subtype of Cushing's syndrome).[1][2] It specifically acts as a potent and selective inhibitor of aldosterone synthase (CYP11B2) and at higher dosages of 11β-hydroxylase (CYP11B1).[2] The drug was also under development for the treatment of heart failure, hypertension, and solid tumors, but development was discontinued for these indications.[1] As of 2017, osilodrostat is in phase III and phase II clinical trials for treatment of pituitary ACTH hypersecretion and Cushing's syndrome, respectively.[1]
 | |
| Clinical data | |
|---|---|
| Other names | LCI-699 |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
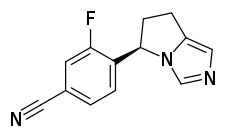
| Formula | C13H10FN3 |
| Molar mass | 227.242 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- http://adisinsight.springer.com/drugs/800026342
- Fleseriu M, Castinetti F (2016). "Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies". Pituitary. 19 (6): 643–653. doi:10.1007/s11102-016-0742-1. PMC 5080363. PMID 27600150.